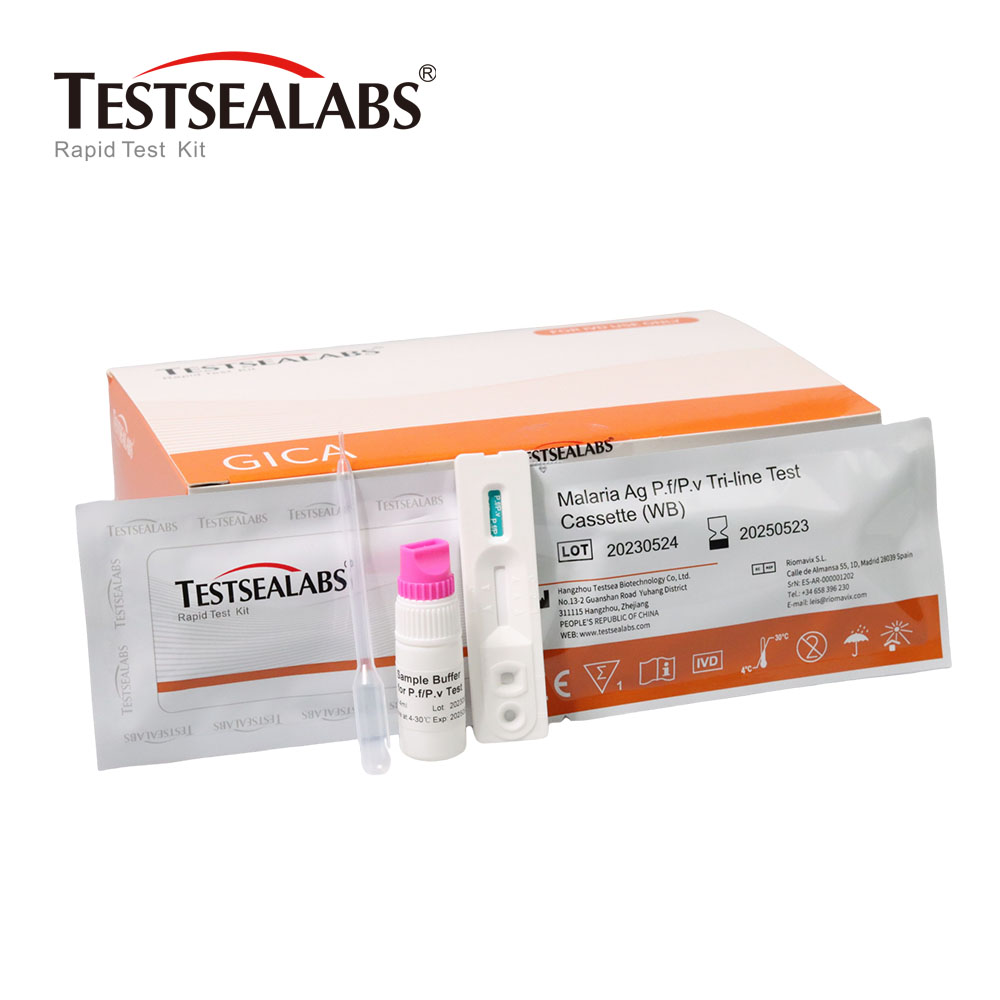
Testsealabs Malaria Ag Pf/Pv Tri-line Test Cassette
Quick Details
| Zita reBrand: | testsea | Product name: | Malaria pf/pan tri-line test kit |
| Nzvimbo Yekwakabva: | Zhejiang, China | Type: | Pathological Analysis Equipments |
| Chitupa: | ISO9001/13485 | Instrument classification | Kirasi II |
| Kururama: | 99.6% | Muenzaniso: | Ropa rose |
| Format: | Cassete/Mutsetse | Tsanangudzo: | 3.00mm/4.00mm |
| MOQ: | 1000 Pcs | Sherufu hupenyu: | 2 years |
Chinangwa Kushandiswa
Malaria antigen pf kukurumidza bvunzo ndeye immunochromatography yakavakirwa pane imwe-nhanho in vitro diagnostic bvunzo yekumisikidza kwePf/pan muropa remunhu serubatsiro mukuongororwa kwechirwere cheMalaria.

Summary
Malaria inokonzerwa neutachiona hunonzi Plasmodium, hunotapurirwa nekurumwa neumhutu hune utachiona. Zviratidzo zvemarariya zvinosanganisira kupisa muviri, kutemwa nemusoro, uye kurutsa, uye zvinowanzoonekwa mazuva ari pakati pe10 ne15 pashure pokunge umhutu hwarumwa. Kana ikasarapwa, marariya inogona kukurumidza kuuraya nokukanganisa kufambiswa kweropa kunhengo dzinokosha. Munzvimbo dzakawanda dzenyika, utachiona hwacho hwakatanga kusakunda mishonga yakawanda yemarariya.
Test Procedure
Bvumira bvunzo, sampuli, buffer uye/kana zvinodzora kusvika mukamuri tembiricha 15-30 ℃ (59-86 ℉) isati yaedzwa.
1. Hunza homwe yacho kune tembiricha yemumba usati waivhura. Bvisa mudziyo wekuyedza kubva kuhomwe yakavharwa uye ishandise nekukurumidza sezvinobvira.
2. Isa mudziyo wekuedza panzvimbo yakachena uye yakaenzana.
3. Yeserum kana plasma specimen: Bata chinodonhedza chakatwasuka wotamisa madonhwe matatu eserum.kana plasma (inenge 100μl) kune specimen tsime (S) yechishandiso chekuyedza, wobva watangatimer. Ona mufananidzo uri pasi apa.
4. Kune zvienzaniso zveropa rose: Bata chinodonhedza pasi uye utumidze 1 donho rero roseropa (rinenge 35μl) kune yemuenzaniso mutsime (S) yebvunzo mudziyo, wobva wawedzera madonhwe maviri ebhafa (anenge 70μl) uye tanga iyo timer. Ona mufananidzo uri pasi apa.
5. Mirira kuti mutsara wemavara (ma) uoneke. Verenga zvawanikwa pamaminitsi gumi nemashanu. Usadudzire themhedzisiro mushure memaminitsi makumi maviri.
Kushandisa huwandu hwakakwana hwemuenzaniso kwakakosha kune mhedzisiro yebvunzo. Kana kutama (the wettingye membrane) haina kucherechedzwa muhwindo rekuyedza mushure meminiti imwe, wedzera rimwe donhwe rebuffer(yeropa rose) kana sampuli (yeserum kana plasma) kune tsime remuenzaniso.
Dudziro yeZvabuda
Zvakanaka:Mitsetse miviri inooneka. Mutsetse mumwe unofanirwa kugara uchionekwa munharaunda yekutonga (C), uyemumwe mutsara unooneka une ruvara unofanirwa kuoneka munharaunda yebvunzo.
Negative:Mutsetse mumwe uneruvara unoonekwa munzvimbo yekudzora(C).Hapana mutsara unoonekera une ruvara unoonekadunhu rebvunzo.
Hazvina basa:Mutsetse wekudzora watadza kuoneka. Kusakwana kuenzanisa vhoriyamu kana kuti maitiro asiri iwomatekiniki ndiwo anonyanya zvikonzero zvekutadza kutonga mutsetse.
★ Ongorora maitiro uye dzokororabvunzo nechishandiso chitsva chekuyedza. Kana dambudziko rikaramba riripo, regedza kushandisa test kit nekukasira uye ubate mushambadzi wenzvimbo yako.
Exhibition Information






Profile yekambani
Isu, Hangzhou Testsea Biotechnology Co., Ltd ikambani inokura nekukurumidza nyanzvi yebiotechnology nyanzvi mukutsvaga, kugadzira, kugadzira uye kugovera epamberi in-vitro diagnostic (IVD) bvunzo kits uye zviridzwa zvekurapa.
Nzvimbo yedu ndeye GMP, ISO9001, uye ISO13458 certified uye isu tine CE FDA mvumo. Ikozvino tave kutarisira kushanda pamwe nemamwe makambani ari mhiri kwemakungwa mukusimudzirana.
Isu tinogadzira bvunzo dzekubereka, bvunzo dzezvirwere zvinotapukira, bvunzo dzekushandisa zvisina kunaka zvinodhaka, bvunzo dzemwoyo, bvunzo dze tumor marker, chikafu uye chengetedzo bvunzo uye bvunzo dzechirwere chemhuka, uyezve, mhando yedu TESTSEALABS yave ichizivikanwa mumisika yemumba neyekunze. Yakanakisa mhando uye mitengo yakanaka inoita kuti titore pamusoro pe50% migove yemumba.
Product Process

1.Gadzirira

2.Chivharo

3.Muchinjikwa membrane

4.Cheka tambo

5.Assembly

6.Pack the pouches

7.Sima zvikwama

8.Pack bhokisi

9.Encasement