The Vibro Cholerae O139(VC O139) and O1(VC O1) Combo Test utilizes an immunochromatography technique to identify two significant strains of cholera bacteria. This test is crucial for timely cholera detection, allowing health authorities to implement swift interventions. Effective use of the Vibro Cholerae O139(VC O139) and O1(VC O1) Combo enhances outbreak management, ultimately reducing morbidity and mortality rates associated with cholera.
| Year | Cases Reported | Deaths Reported | Change in Deaths |
|---|---|---|---|
| 2023 | 535,321 | 4,000 | +71% |
Key Takeaways
- The Vibro Cholerae O139 and O1 Combo Test allows for rapid detection of cholera strains, enabling quick public health responses.
- Effective sample collection and proper testing procedures are crucial for accurate cholera diagnosis and outbreak management.
- Recent innovations in testing, such as rapid diagnostic tests, significantly improve detection speed and enhance cholera surveillance efforts.
Methodology of the Vibro Cholerae O139 and O1 Combo Test Immunochromatography Technique
Sample Collection Techniques
Effective sample collection is vital for accurate cholera testing. Health professionals should follow specific protocols to ensure the integrity of the samples. Recommended practices include:
- Stool Specimens: Collect 4 to 10 stool samples from patients suspected of cholera. These samples must be sent to a microbiology laboratory for confirmation, strain identification, and antibiotic sensitivity assessment.
- Transport Media: Confirm the preferred transport media with the laboratory. Options may include filter paper or Cary-Blair, which help preserve the viability of the samples during transport.
Testing Procedures
The Vibro Cholerae O139(VC O139) and O1(VC O1) Combo Test utilizes an immunochromatography technique that allows for rapid detection of cholera strains. The following equipment and reagents are essential for conducting the test:
| Equipment/Reagents | Description |
|---|---|
| StrongStep® Vibrio cholerae O1/O139 Antigen Combo Rapid Test | A rapid visual immunoassay for the qualitative detection of Vibrio cholerae O1 and/or O139 in human fecal specimens. |
| Anti-Vibrio cholerae O1/O139 antibodies | Immobilized on the test region of the membrane for detection. |
| Colored particles | Conjugated to antibodies for visual interpretation of results. |
| Specimen | Human fecal specimens, which must be tested immediately after collection. |
| Storage conditions | Store kit at 4-30°C, do not freeze, and protect from contamination. |
The testing process involves applying the stool sample to the test device, where it interacts with the antibodies. A visible line indicates the presence of the cholera bacteria, allowing for quick diagnosis.
Sensitivity and Specificity
The sensitivity and specificity of the Vibro Cholerae O139 and O1 Combo Test are critical metrics for evaluating its effectiveness. Recent clinical studies report the following rates:
| Test Type | Sensitivity | Specificity |
|---|---|---|
| V. cholerae O139 (filtered samples) | 1.5 × 10² CFU/ml | 100% |
| V. cholerae O139 (unfiltered samples) | One log lower than filtered | 100% |
Additionally, pooled sensitivity and specificity for cholera rapid diagnostic tests show:
| Test Type | Pooled Sensitivity | Pooled Specificity |
|---|---|---|
| Cholera Rapid Diagnostic Tests | 90% (86% to 93%) | 91% (87% to 94%) |
These high rates indicate that the Vibro Cholerae O139(VC O139) and O1(VC O1) Combo Test Immunochromatography technique provides reliable results, making it a valuable tool in cholera detection and outbreak management.
Importance in Public Health

Role in Outbreak Management
The Vibro Cholerae O139 and O1 Combo Test plays a pivotal role in managing cholera outbreaks. Rapid detection of cholera strains allows health authorities to implement timely interventions. This test significantly enhances the speed and effectiveness of public health responses.
- Increased Screening: The introduction of rapid diagnostic tests (RDTs) has led to increased screening for cholera. Communities previously thought to be free of cholera now show cases due to improved detection capabilities.
- Cost-Effectiveness: RDTs are more cost-effective and less time-consuming than traditional laboratory tests. This efficiency facilitates quicker diagnosis and treatment, which is crucial during outbreaks.
- Immediate Results: New rapid tests provide results in minutes, significantly faster than traditional lab tests that can take days. This quick turnaround is essential for preventing further infections and initiating timely vaccination campaigns.
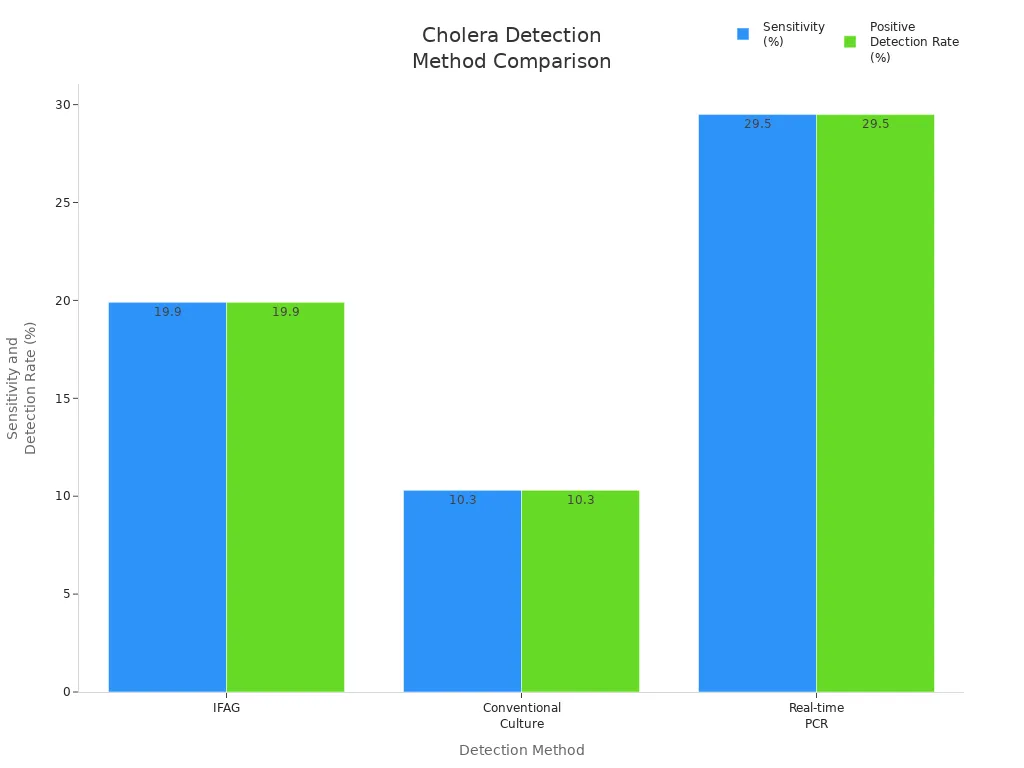
The following table illustrates the sensitivity and positive detection rates of various cholera detection methods, highlighting the advantages of the Vibro Cholerae O139 and O1 Combo Test:
| Method | Sensitivity (%) | Specificity (%) | Positive Detection Rate (%) |
|---|---|---|---|
| IFAG | 19.9 | High | 29/146 |
| Conventional Culture | 10.3 | Lower | 15/146 |
| Real-time PCR | 29.5 | Highest | 43/146 |
Case Studies of Effective Use
Case studies demonstrate the effectiveness of the Vibro Cholerae O139 and O1 Combo Test in various regions. For instance, research indicates significant differences in antibiotic resistance rates between Vibrio cholerae O139 and O1 strains. O1 strains are often linked to large outbreaks, while O139 strains tend to be associated with sporadic cases and foodborne outbreaks. Understanding these patterns is crucial for managing cholera epidemics, particularly in vulnerable areas like rural Bangladesh.
Global Health Implications
The global burden of cholera remains significant, affecting approximately 1.3 billion people, with the majority of cases concentrated in sub-Saharan Africa and South Asia. Outbreaks often become extensive and prolonged, as evidenced in countries such as Yemen and Haiti. Traditional gold-standard diagnostic methods, including microbial culture and PCR, require considerable time, trained personnel, and laboratory infrastructure, often leading to delays in outbreak confirmation and response. These limitations contribute to increased morbidity and mortality and hinder accurate estimation of the cholera burden, placing additional health and economic strain on affected regions.
In this context, immunochromatography-based rapid diagnostic tests (RDTs) offer a transformative approach. By detecting Vibrio cholerae O1 and O139 antigens through lateral flow immunoassays, these tests provide qualitative results within 5 minutes, without requiring cold chain storage or complex equipment. They can be administered with minimal training at the point of care, making them particularly valuable in remote and low-resource settings. Although not intended for definitive patient diagnosis, RDTs have high negative predictive value, reducing the need for confirmatory tests in low-prevalence areas. Their primary application lies in epidemiological surveillance, where their speed and cost-effectiveness enable early outbreak detection, better monitoring of spatiotemporal trends, and more efficient deployment of interventions such as oral cholera vaccines (OCVs) and sanitation measures—especially critical given the current limited global OCV supply.
The implications of adopting immunochromatography are far-reaching: enhanced real-time surveillance improves prediction accuracy and optimizes outbreak response; standardizing case definitions across countries becomes more feasible with harmonized rapid testing; and the resulting data streams can be integrated with artificial intelligence for deeper analysis of transmission dynamics. Ultimately, these innovations are essential for advancing global cholera control, reducing preventable deaths, and alleviating the health and economic impacts on vulnerable populations.
The Vibro Cholerae O139 and O1 Combo Test plays a vital role in cholera detection. It reliably identifies cholera strains, enabling swift public health responses. With a sensitivity of detecting as few as 103 cells of V. cholerae, this test proves essential in outbreak management.
Increased awareness and utilization of this test among healthcare providers is crucial. The following table highlights the prevalence and antibiotic resistance of cholera serogroups:
| Serogroup | Prevalence (%) | Antibiotic Resistance (%) |
|---|---|---|
| O1 | High | 70% (cefotaxime), 62.4% (trimethoprim-sulfamethoxazole), 56.8% (ampicillin) |
| O139 | Moderate | N/A |
Health authorities must prioritize this test to enhance cholera control efforts globally.
FAQ
What is the primary purpose of the Vibro Cholerae O139 and O1 Combo Test?
The test quickly identifies cholera strains, enabling timely public health interventions.
How long does it take to get results from the Combo Test?
Read results at 5 minutes. Do not interpret the result after 10 minutes.
Yes, the test can simultaneously detect both Vibrio cholerae O1 and O139 strains in a single sample.
Post time: Sep-05-2025


