Beyond Mosquito Nets: Why Post-Protection Testing is Critical in the 2025 Arbovirus Outbreak
Geneva, August 6, 2025 – As the World Health Organization (WHO) warns of accelerating chikungunya outbreaks across 119 countries, health experts are emphasizing a critical gap in mosquito-borne disease prevention: even with rigorous protection measures, post-exposure testing remains essential. Testsealabs, a leading diagnostic solutions provider, highlights its rapid testing kits as a vital tool in combating the overlapping threats of chikungunya, dengue, and Zika viruses.
The Hidden Epidemic: Rising Cases Despite Prevention Efforts
2025 has seen unprecedented arbovirus activity worldwide. The Americas reported over 7 million dengue cases by April alone – a 52% increase from 2023′s annual total (WHO, 2024). Meanwhile, chikungunya has infected 220,000 people across 14 countries in the first half of 2025, with France and Italy documenting their first local transmissions since 2019 (ECDC, 2025).
“People falsely assume mosquito nets and repellents provide complete protection,” says Dr. Elena Rodriguez, infectious disease specialist at the Tropical Medicine Institute. “Our research shows 85% of households use ineffective methods like ultrasonic repellers, while 60% of symptomatic travelers delay testing beyond the critical window.”
The Diagnostic Challenge: When Symptoms Overlap
The clinical similarity between arboviruses creates dangerous delays in treatment. A Honduran study published in PLOS Neglected Tropical Diseases found primary care physicians correctly diagnosed only 30.8% of arbovirus cases, with dengue confirmation rates as low as 8.23%.
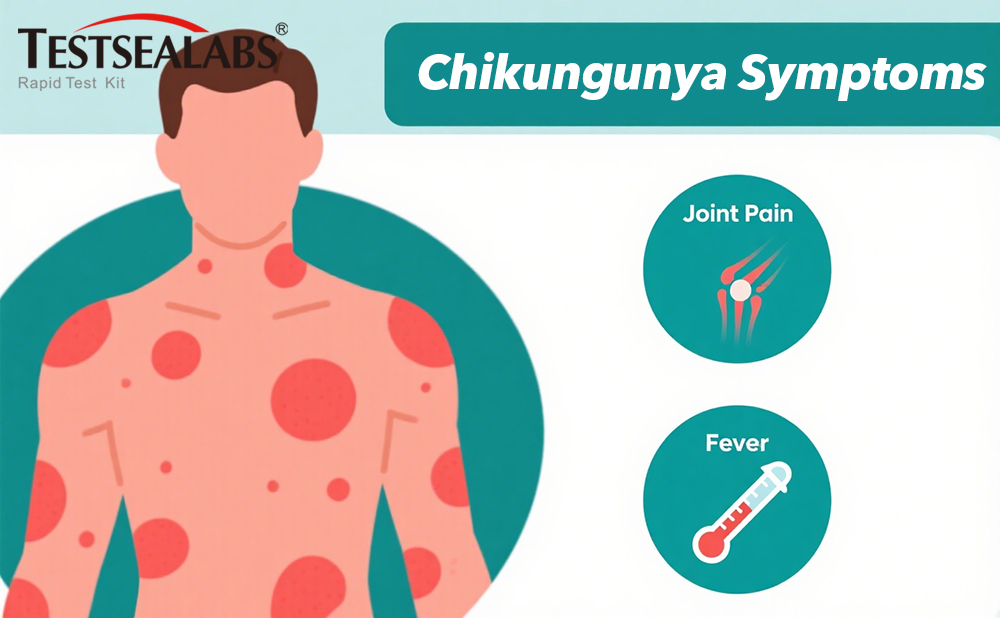
“Chikungunya’s ‘breakbone fever’ shares 80% of symptoms with dengue, including high fever and joint pain,” explains Dr. Rodriguez. “Without testing, even experienced clinicians cannot distinguish these diseases – and misdiagnosis increases severe complication risks by 3.2 times.”
Testsealabs Solutions: Clarity in 15 Minutes
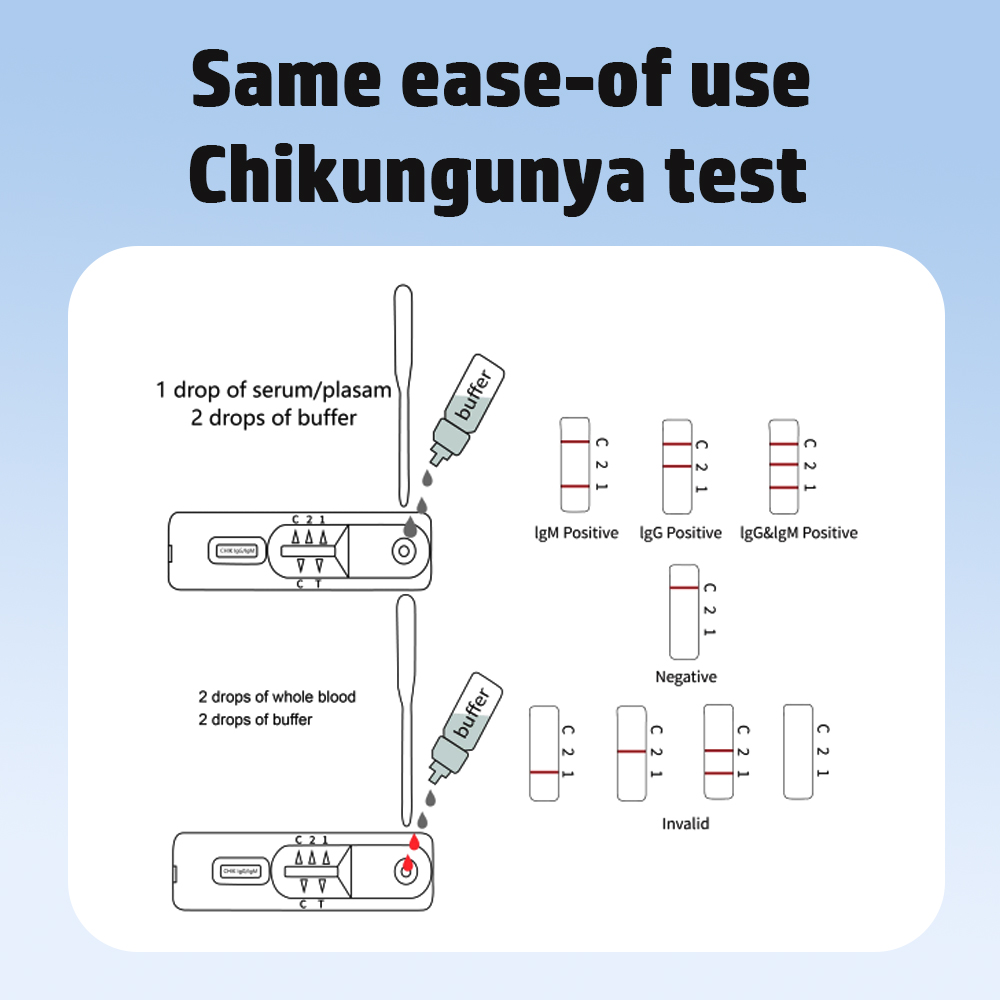
Testsealabs’ arbovirus testing portfolio addresses this critical need with CE-certified, ISO 13485-compliant rapid diagnostic kits designed for both professional and home use:
| Product Name | Detection Targets | Optimal Testing Window | Turnaround Time | Certifications |
| Chikungunya IgM Test | Chikungunya virus-specific IgM antibodies | 5-12 days post-symptoms | 15 minutes | CE, ISO 13485 |
| Dengue IgG/IgM Test | Dengue virus IgG/IgM antibodies | 7+ days post-symptoms | 15 minutes | CE, ISO 13485 |
| Combo Test | Dengue NS1 antigen + Dengue IgG/IgM + Zika/Chikungunya IgG/IgM | Acute to convalescent phase | 15 minutes | CE, ISO 13485 |
The Combo Test enables simultaneous differential diagnosis of co-circulating viruses – a capability deemed “essential” in WHO’s 2024 Global Arbovirus Strategy. Its no-equipment-required design allows results within 15 minutes using whole blood or serum.
Global Health in Your Hands
As climate change expands Aedes mosquito habitats, self-testing becomes a frontline defense,
About Testsealabs
Founded in 2015, Hangzhou Testsea Biotechnology specializes in rapid diagnostic solutions for infectious diseases.
Disclaimer: This press release contains forward-looking statements. Actual results may vary. Testsealabs’ products are for in vitro diagnostic use only. Always consult healthcare professionals for medical advice.
Post time: Aug-06-2025