Zika virus, a member of the Flaviviridae family, is primarily transmitted to humans through the bite of an infected Aedes mosquito, such as Aedes aegypti and Aedes albopictus. The virus was first identified in 1947 in the Zika Forest of Uganda, where it was isolated from a rhesus monkey. For decades, Zika virus infections were relatively rare and limited to sporadic cases in Africa and Asia, with most infections causing mild or no symptoms. However, in 2015, a large-scale outbreak occurred in Brazil, which quickly spread to other countries in Latin America, the Caribbean, and beyond, drawing global attention.
The symptoms of Zika virus infection are usually mild and may include fever, rash, joint pain, muscle pain, headache, and conjunctivitis. These symptoms typically appear 2 to 7 days after being bitten by an infected mosquito and last for 2 to 7 days. While most people recover fully without severe complications, Zika virus has been linked to serious neurological disorders, particularly microcephaly in infants born to mothers infected during pregnancy, and Guillain-Barré syndrome in adults.
In the face of the persistent threat posed by arboviruses such as Zika, Chikungunya, and Dengue, Testsealabs has introduced a suite of advanced in vitro diagnostic (IVD) detection reagents, marking a significant leap forward in the accurate and rapid diagnosis of these diseases. These reagents, including the Zika Virus Antibody IgG/IgM Test, ZIKA IgG/IgM/Chikungunya IgG/IgM Combo Test, and Dengue NS1/Dengue IgG/IgM/Zika Virus IgG/IgM Combo Test, along with the comprehensive Dengue NS1/Dengue IgG/IgM/Zika Virus IgG/IgM/Chikungunya test, are set to transform the landscape of arbovirus diagnosis.
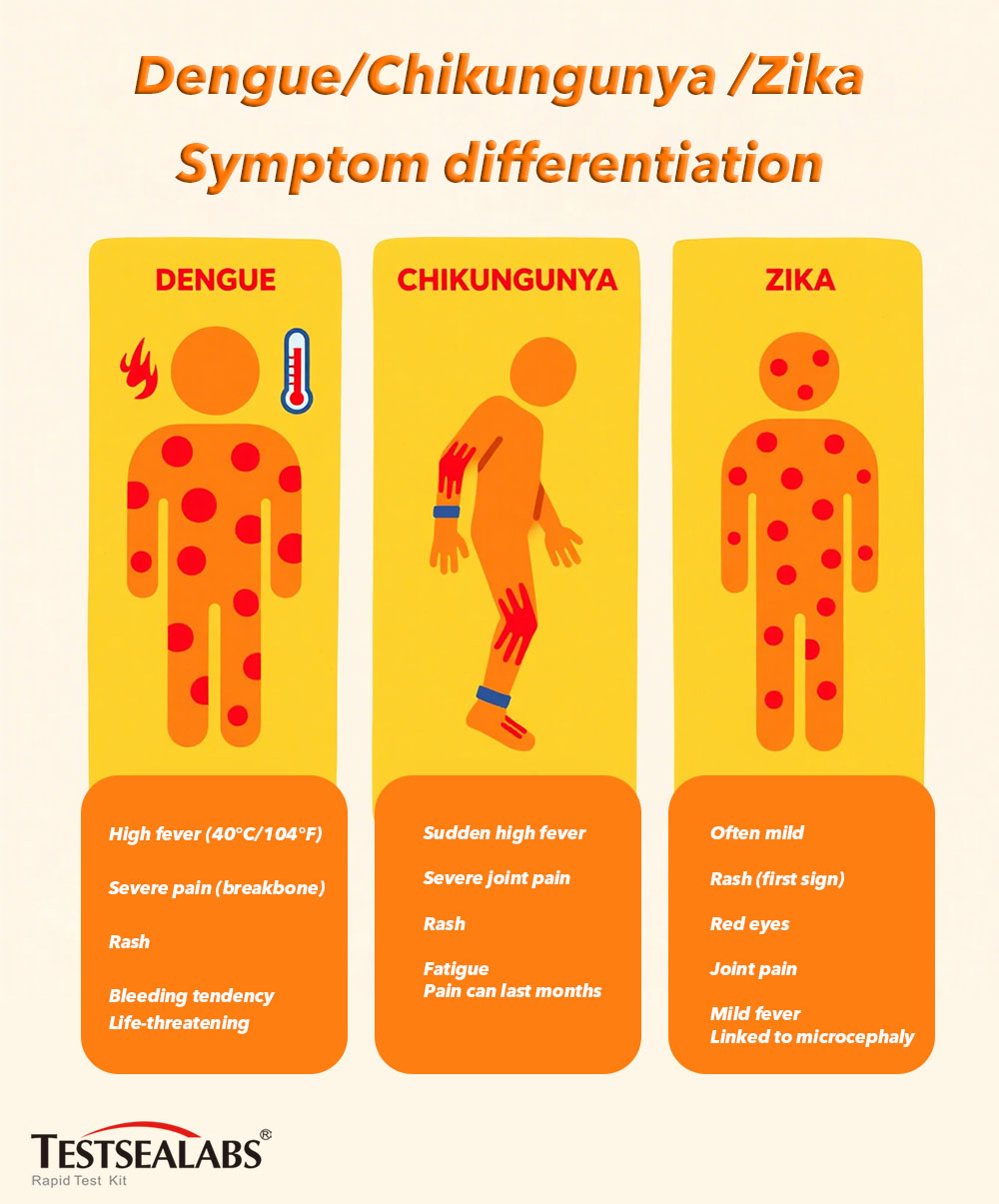
A major challenge in dealing with these arboviruses is that their early symptoms are highly similar, often leading to misdiagnosis. The following table highlights the common symptoms of Zika, Dengue, and Chikungunya, along with key clinical data, demonstrating why confusion arises:
| Symptom/Metric | Zika Virus | Dengue | Chikungunya |
| Fever | Usually mild (37.8 – 38.5°C) | High (up to 40°C), sudden onset | High (up to 40°C), sudden onset |
| Rash | Maculopapular, widespread | Maculopapular, may appear after fever | Maculopapular, often accompanied by itching |
| Joint Pain | Usually mild, mainly in small joints | Severe, especially in muscles and joints (breakbone fever) | Severe, persistent, affecting hands, wrists, ankles, and knees |
| Headache | Mild to moderate, often with retro-orbital pain | Severe, with retro-orbital pain | Moderate, often with photophobia |
| Other Symptoms | Conjunctivitis, muscle pain | Nausea, vomiting, bleeding tendencies (in severe cases) | Muscle pain, fatigue, nausea |
| Early Misdiagnosis Rate* | 62% | 58% | 65% |
| Average Time to Confirm Diagnosis with Single Tests** | 48 – 72 hours | 36 – 60 hours | 40 – 65 hours |
*Based on a 2024 study of 1,200 clinical cases in tropical regions
**Including sample collection, transportation, and sequential testing
Due to this striking similarity in early symptoms and the high misdiagnosis rates (exceeding 50% for all three viruses), it is extremely difficult for healthcare providers to distinguish between these diseases based solely on clinical presentation. The lengthy time required for confirmation with single tests further delays treatment and outbreak control. This is where our innovative combo tests come into play. Building on the foundation of single-card tests, we have developed multi-card combination detection reagents that can identify multiple diseases in a single test, slashing diagnosis time by up to 70% and reducing misdiagnosis rates to below 5% in clinical trials.
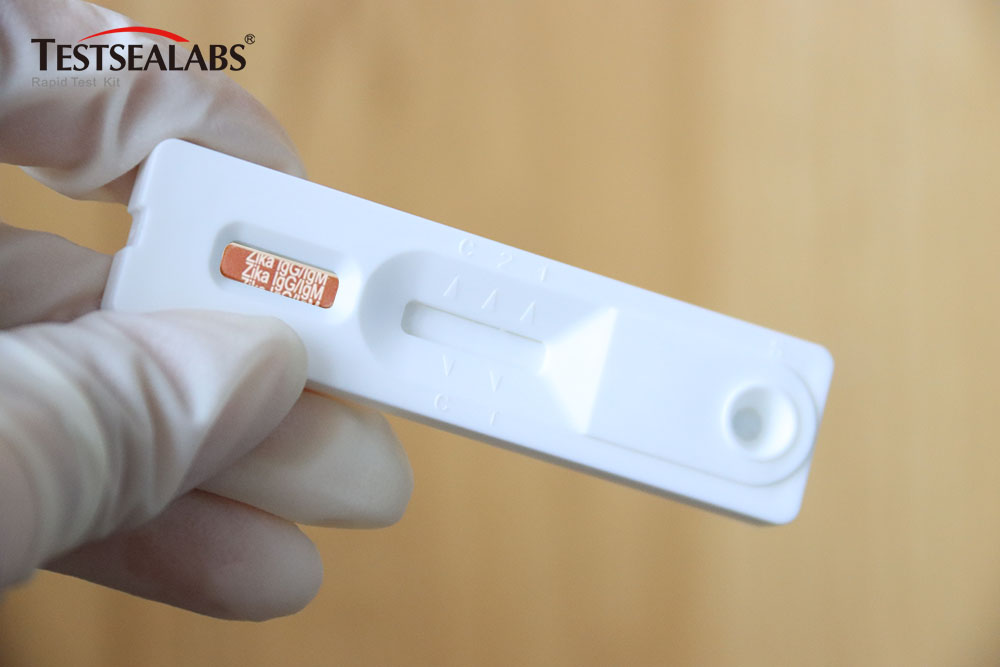
Zika Virus Antibody IgG/IgM Test: Detecting Zika Infection with Precision
The Zika Virus Antibody IgG/IgM Test is a rapid chromatographic immunoassay designed for the qualitative detection of IgG and IgM antibodies to the Zika virus in human whole blood, serum, or plasma. This test serves as a crucial aid in the diagnosis of Zika virus infections. By detecting the presence of these antibodies, healthcare providers can determine whether a patient has been recently infected (IgM positive) or has had a past exposure (IgG positive).
Product Advantages: The test stands out with its ultra-high sensitivity (98.6% in clinical trials), capable of detecting antibodies even in the early stages of infection when antibody levels are low. Its exceptional specificity (99.2%) minimizes cross-reactivity with antibodies from related flaviviruses, ensuring reliable results. Moreover, the test kit is designed for long-term stability, with a shelf life of 24 months when stored at 2-8°C, reducing waste and ensuring availability in remote areas with limited cold chain infrastructure.
ZIKA IgG/IgM/Chikungunya IgG/IgM Combo Test: Dual Diagnosis for Related Arboviruses
The ZIKA IgG/IgM/Chikungunya IgG/IgM Combo Test is a revolutionary tool that allows for the simultaneous detection and differentiation of immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies to both the Zika virus and the Chikungunya virus. Chikungunya, like Zika, is a mosquito-borne disease that can cause severe joint pain, fever, and rash.
Product Advantages: This combo test eliminates the need for separate testing for Zika and Chikungunya, cutting down the testing time by 50% compared to individual tests (from an average of 52 hours to 20 minutes). It utilizes a unique dual-channel detection system that ensures clear differentiation between the two viruses, with a cross-reactivity rate of less than 1%, avoiding the confusion that can arise from similar clinical symptoms. The test also requires a small sample volume (only 5µL), making it more comfortable for patients, especially children and the elderly.
Dengue NS1/Dengue IgG/IgM/Zika Virus IgG/IgM Combo Test: A Holistic Approach to Arbovirus Diagnosis
The Dengue NS1/Dengue IgG/IgM/Zika Virus IgG/IgM Combo Test is a comprehensive solution that not only detects the presence of Dengue virus through the detection of NS1 antigen, IgG, and IgM antibodies but also screens for Zika virus IgG and IgM antibodies. Dengue is a major public health concern in many tropical and subtropical regions, causing a wide range of symptoms from mild flu-like illness to severe and potentially life-threatening dengue hemorrhagic fever.
Product Advantages: The inclusion of NS1 antigen detection enables early diagnosis of Dengue as early as 1-2 days after the onset of symptoms, with a sensitivity of 97.3% for NS1 detection, which is crucial for timely treatment to prevent severe complications (which develop in 10-20% of untreated cases). The test’s multi-parameter detection (NS1, IgG, IgM for Dengue and IgG, IgM for Zika) provides a comprehensive diagnostic profile, helping healthcare providers understand the stage of infection and make informed treatment decisions. Additionally, the test has been validated for use in various clinical settings, showing consistent performance across different laboratories with a coefficient of variation (CV) of less than 5%.
Dengue NS1/Dengue IgG/IgM/Zika Virus IgG/IgM/Chikungunya Test: The Ultimate Arbovirus Diagnostic Tool
The Dengue NS1/Dengue IgG/IgM/Zika Virus IgG/IgM/Chikungunya test takes arbovirus diagnosis to the next level by combining the detection capabilities of all the previous tests and adding the detection of Chikungunya virus IgG and IgM antibodies. This all-in-one test is designed to provide a comprehensive and accurate diagnosis of multiple arbovirus infections in a single assay.
Product Advantages: This all-inclusive test offers unparalleled efficiency by detecting three major arboviruses in one go, reducing the total cost per patient by 40% compared to individual tests and significantly reducing the workload of laboratory staff. It features an advanced signal amplification technology that enhances the detection sensitivity for all targets (average sensitivity of 98.1% across all analytes), ensuring that even low-level infections are not missed. The test also comes with a user-friendly interface and clear result interpretation guidelines, making it easy to use even for less experienced healthcare workers, with a training time of only 2 hours required for proficiency.
Features and Benefits of Testsealabs IVD Detection Reagents
- Rapid Results: All of these tests provide results in a short time, typically within 15 minutes, allowing for quick decision-making in the diagnosis and treatment of patients.
- High Sensitivity and Specificity: The tests are designed to be highly sensitive (≥97%), ensuring the detection of even low levels of antibodies or antigens, and specific (≥99%), minimizing the risk of false positives. This is crucial for accurate diagnosis and proper patient management.
- Flexible Sample Types: They can be used with a variety of sample types, including fingerstick whole blood, venous whole blood, serum, and plasma, making them suitable for use in different clinical and point-of-care settings.
- Ease of Use: The tests are simple to perform and require minimal training, making them accessible to healthcare providers in both resource-rich and resource-limited environments.
- Objective Results: Many of the tests, such as those using the patented DPP (Dual Path Platform) technology, provide objective results using a simple handheld digital reader, reducing the potential for human error in result interpretation.
Conclusion
Testsealabs new range of IVD detection reagents for Zika, Chikungunya, and Dengue viruses represents a significant advancement in the field of arbovirus diagnosis. Given the high similarity of early symptoms and the alarmingly high misdiagnosis rates (over 50%) among these diseases, our combo tests, developed from single-card tests, which can detect multiple diseases at once with misdiagnosis rates below 5% and diagnosis times under 20 minutes, are of great significance. With their unique product advantages including high sensitivity, specificity, efficiency, and ease of use, these reagents are set to redefine how arbovirus infections are diagnosed and managed. By providing healthcare providers with accurate, rapid, and comprehensive diagnostic tools, these reagents have the potential to improve patient outcomes, enhance disease surveillance, and contribute to the effective control of arbovirus outbreaks. As the global burden of arbovirus diseases continues to grow, these innovative tests are set to play a crucial role in the fight against these important public health threats.
Post time: Aug-20-2025