Nipah Virus Outbreak Updates: India & Bangladesh Cases Surge, Global Health Alert Activated
Recently, the Nipah virus outbreak has intensified in high-risk regions including West Bengal (India) and Dhaka (Bangladesh), with multiple new confirmed cases reported. Disturbingly, several healthcare workers are among the infected—underscoring the virus’s potential for human-to-human transmission in close-contact settings like hospitals. To date, nearly 100 close contacts have been placed under emergency quarantine, and local governments have activated public health emergency protocols to contain the spread.
Classified as a top-priority zoonotic virus by global health organizations, Nipah virus poses an existential threat due to three critical factors: complex transmission pathways, a 40%-75% case fatality rate, and the absence of specific vaccines or curative treatments. For these reasons, rapid, accurate detection has become the linchpin of effective epidemic prevention and control worldwide.
In response to this global crisis, Testsealabs (Hangzhou Testsea Biotechnology Co., Ltd.) has swiftly developed the Nipah Virus Nucleic Acid Detection Kit (PCR-Fluorescence Probing). Built on advanced molecular diagnostic technology, this kit delivers unparalleled precision and reliability, serving as a core technical pillar for global port quarantine, CDC surveillance, clinical diagnosis, and virus traceability. It stands as a vital tool to fortify the first line of defense against the Nipah virus outbreak.
Key Facts About Nipah Virus: Transmission, Symptoms & Global Risks
1. Natural Hosts and Transmission Routes
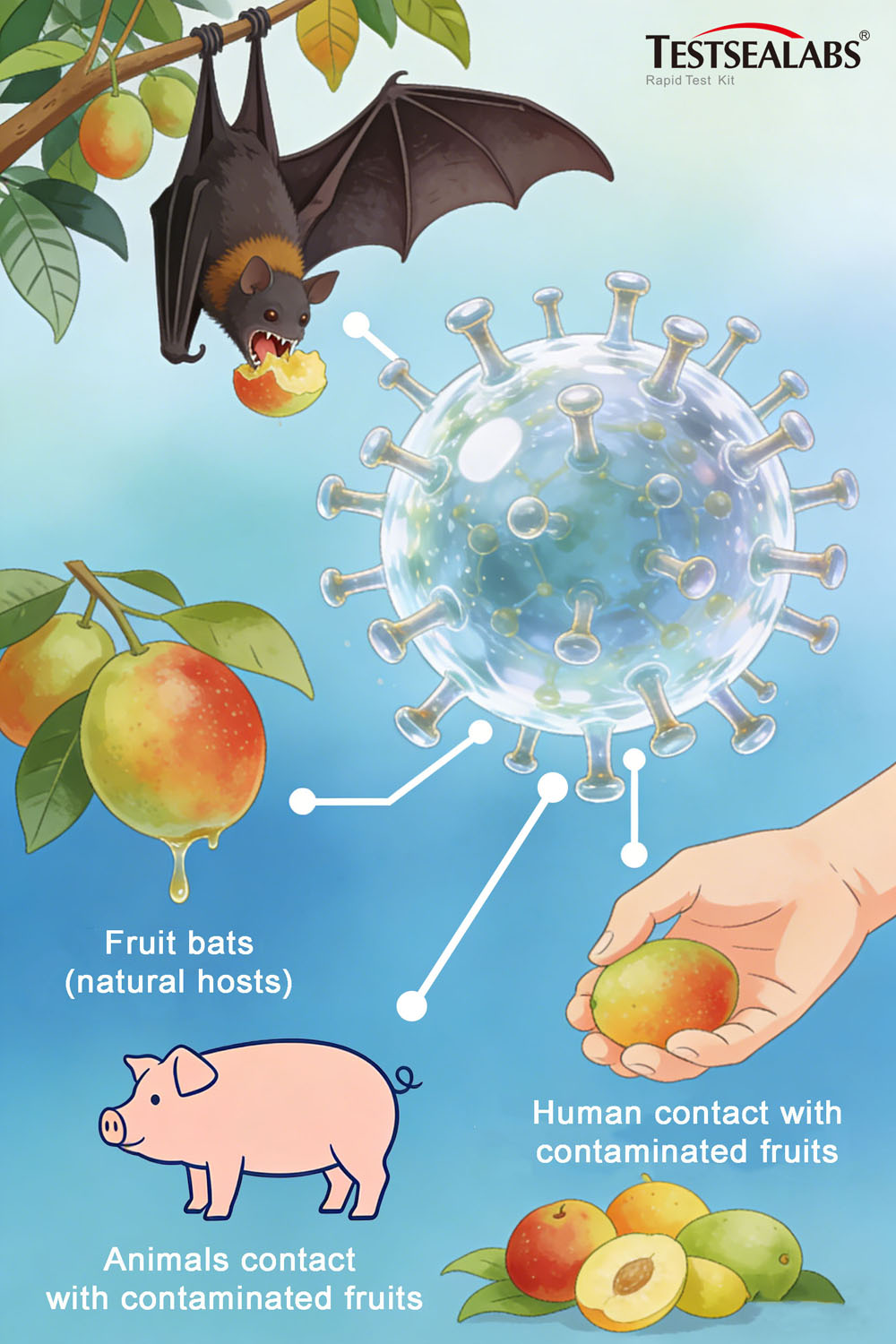
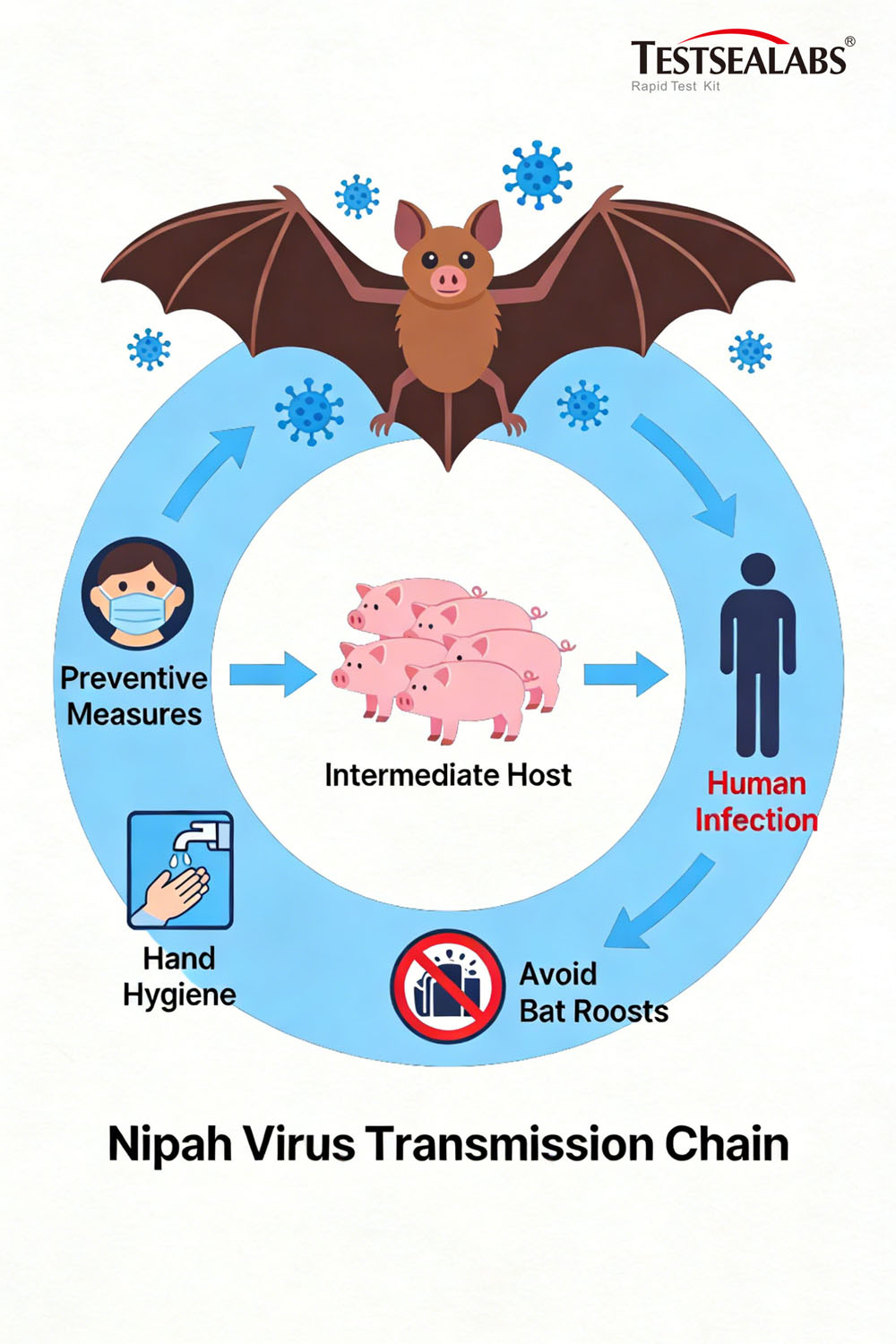
Per authoritative data from the World Health Organization (WHO), the primary natural host of Nipah virus is fruit bats belonging to the Pteropodidae family (flying foxes). Infected bats exhibit no visible symptoms but shed the virus through urine, saliva, and other secretions. Human infection occurs via three main pathways:
- Animal-to-human transmission: Contact with infected domestic animals (e.g., pigs, horses, goats) or consumption of fruits/date palm sap contaminated by bat excreta.
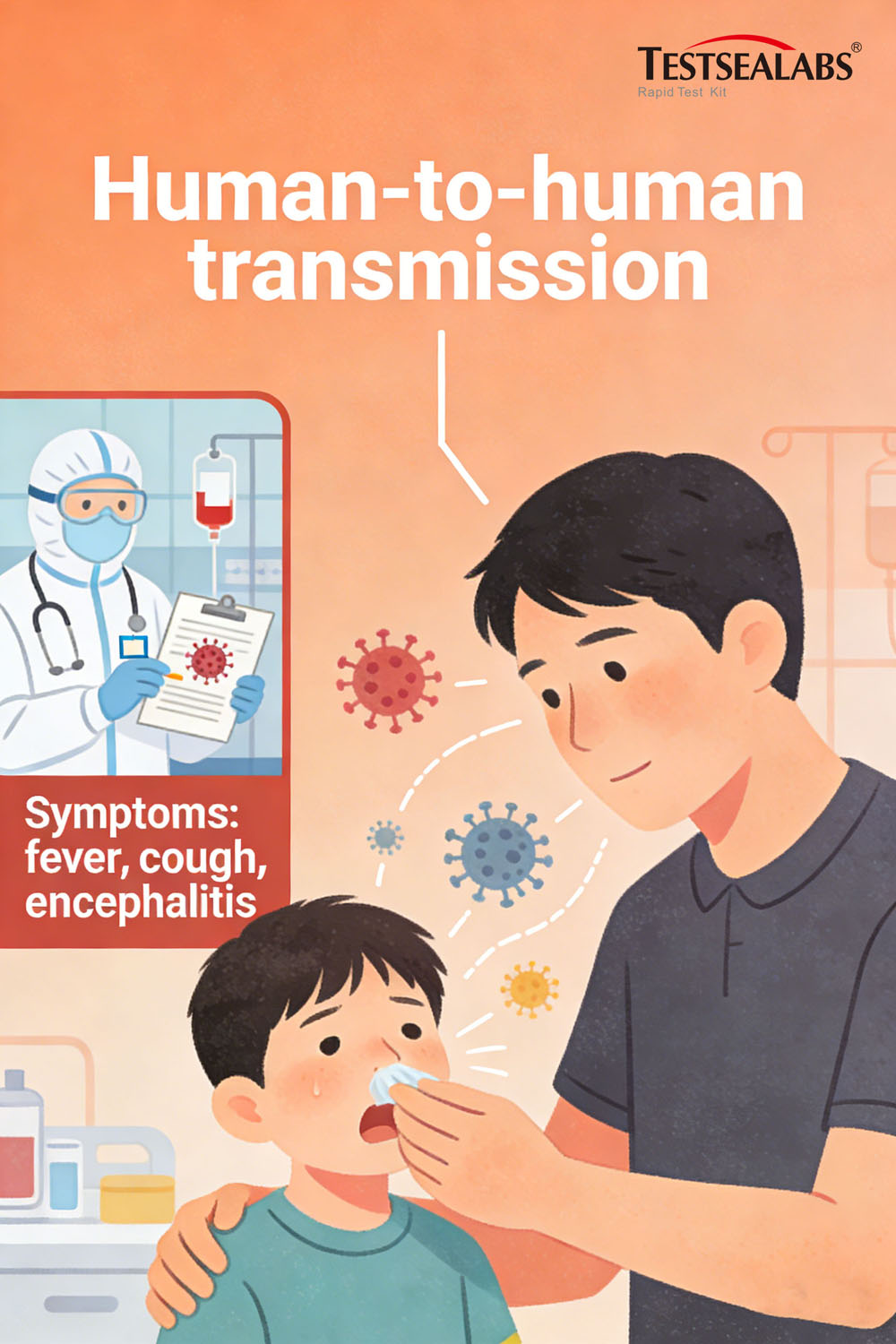
- Human-to-human transmission: Close contact with the secretions, excreta, or respiratory droplets of infected individuals—particularly in enclosed environments such as hospitals and households.
Notable outbreaks: In 2001, a hospital-acquired Nipah cluster in Siliguri (India) affected 75% of healthcare workers and visitors. Between 2001–2008, nearly half of Bangladesh’s reported cases stemmed from caregiving-related human-to-human transmission, emphasizing the urgent need for rapid infection source identification.
2. Clinical Symptoms: Misleading Early Signs and Rapid Progression
Nipah virus infection is notoriously difficult to diagnose in its early stages, as initial symptoms mirror common respiratory illnesses:
- Early symptoms: Fever, headache, muscle pain (myalgia), vomiting, and sore throat.
- Severe progression: Within 24–48 hours, critical cases develop neurological symptoms (dizziness, drowsiness, confusion, acute encephalitis, seizures) and respiratory failure (acute respiratory distress), often leading to coma and death.
- Incubation period: 4–14 days (up to 45 days in rare instances). Asymptomatic carriers during this period may still transmit the virus, creating significant barriers to effective screening and containment.
3. Economic Impact and Global Spread Risks
Beyond its devastating human toll, Nipah virus inflicts severe damage on the livestock industry. During the first documented outbreak in Malaysia (1999), pigs acted as the primary transmission vector. Infected pigs displayed acute fever, breathing difficulties, and neurological symptoms, prompting mass culling that caused catastrophic economic losses for farmers. The virus subsequently spread to Singapore, triggering a cross-border public health emergency.
Today, the virus’s reach extends beyond Asia’s historically affected nations (Malaysia, Bangladesh, India). Nipah virus antibodies have been detected in fruit bat populations in Cambodia, Ghana, Indonesia, Thailand, and other countries—signaling latent outbreak risks on a global scale.
Testsealabs Nipah Virus PCR Detection Kit: A Game-Changer for Rapid, Accurate Diagnosis
Traditional Nipah virus detection methods are hampered by their reliance on specialized laboratories, complex workflows, and lengthy turnaround times—failing to meet the urgent demands of sudden outbreaks for fast case identification and traceability.
With decades of expertise in in vitro diagnostics, Testsealabs has addressed this gap with its independently developed Nipah Virus Nucleic Acid Detection Kit (PCR-Fluorescence Probing). Key advantages of the kit include:
- Targeted precision: Engineered with specific primers and fluorescent probes that bind to the highly conserved region of the Nipah virus genome, ensuring industry-leading sensitivity and specificity.
- Versatile sample compatibility: Accurately detects viral nucleic acids in throat swabs, urine, and cerebrospinal fluid—minimizing the risk of misdiagnosis or missed cases.
- Global compliance: Adheres to WHO and international testing standards, validated through multiple rounds of clinical trials to guarantee stable, reliable performance in diverse settings.
“The core of Nipah virus prevention and control lies in ‘early detection, early isolation, and early intervention’—and accurate detection is the foundation of this strategy,” stated a Testsealabs R&D director. “Our PCR detection kit provides a fast, trustworthy solution for healthcare providers, CDC teams, and port authorities worldwide, enabling them to respond swiftly to outbreaks and save lives.”
Testsealabs’ Commitment to Global Epidemic Response
To meet surging global demand, Testsealabs has activated an emergency production plan to ensure unwavering supply capacity. The company stands ready to rapidly fulfill procurement requests from medical institutions, health departments, port quarantine agencies, and research institutions worldwide—delivering timely technical support for Nipah virus prevention and control.
As the risk of cross-border Nipah virus transmission persists, strengthening detection capabilities has become a global consensus. Testsealabs remains dedicated to leveraging its technical expertise to optimize product performance, expand international cooperation, and develop high-quality solutions for emerging infectious disease control. Guided by the mission “Empowering Epidemic Prevention with Technology, Safeguarding Public Health,” Testsealabs will continue collaborating with global healthcare workers and researchers to build a resilient public health security network.
Post time: Jan-27-2026