
Key Exhibition Information
Exhibition Name: 2025 MEDICA (International Medical Fair Düsseldorf)
Exhibition Time: November 17-20, 2025
Exhibition Venue: Stockumer Kirchstraße 61, D-40474 Düsseldorf, Germany
Testsealabs Booth No.: 3H19
Düsseldorf, Germany, November 17, 2025 —— The 2025 MEDICA (International Medical Fair Düsseldorf), the annual grand event of the global medical industry, officially kicked off on November 17th. As an innovative high-tech enterprise specializing in in vitro diagnostics (IVD) in China, Hangzhou Testsea Biotechnology Co., Ltd. (hereinafter referred to as “Testsealabs”) made its debut at Booth 3H19 with three core product lines: women’s health testing, gastrointestinal disease screening, and respiratory pathogen detection. With its precise and efficient testing solutions and cutting-edge technological strength, Testsealabs has become one of the highlights of the exhibition.
Global Medical Feast Supports, Chinese IVD Innovation Attracts Attention

As the world’s largest and most influential comprehensive medical exhibition, this year’s MEDICA is themed “Smart Interconnection · Healthy Future”, covering 16-18 exhibition halls with an exhibition area of 150,000 square meters, gathering top medical enterprises and innovative achievements from more than 130 countries and regions. During the exhibition, several professional forums such as MEDICA LABMED FORUM were held, focusing on cutting-edge fields such as in vitro diagnostics, digital medical care and sustainable medical care, building an efficient technical exchange and business cooperation platform for the global medical industry chain.

Founded in November 2015 and headquartered in Yuhang District, Hangzhou, Hangzhou Testsea Biotechnology Co., Ltd. (Testsealabs for short) is a national high-tech enterprise focusing on the R&D, production and sales of IVD reagents. It is also a key “large enterprise and group” cultivated by Yuhang District, as well as a “specialized, refined, distinctive and innovative” enterprise in Zhejiang Province and a provincial enterprise research institute in Zhejiang. With profound technical accumulation and innovative capabilities, Testsealabs has quickly emerged as an outstanding player in the IVD industry.
Three Core Product Matrix, Solving Clinical Testing Pain Points
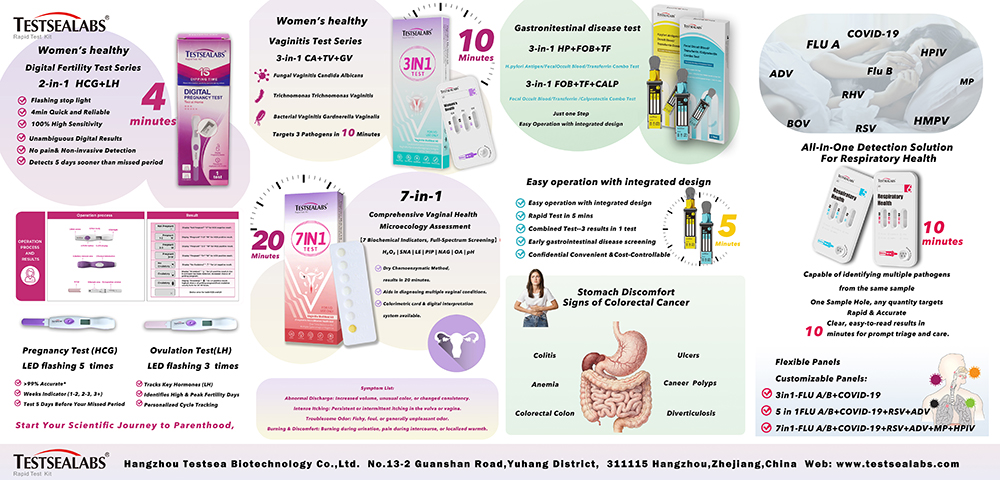
At this exhibition, Testsealabs closely aligned with global clinical needs and focused on showcasing three series of innovative products. With the core advantages of “fast, accurate and simple”, the products solve industry pain points and demonstrate the technological innovation strength of Chinese IVD enterprises.
Women’s Health Testing Area: Full-Cycle Protection for “Her Health”
Focusing on women’s fertility planning and reproductive health, this section launched three star products to build a full-cycle health protection system from fertility planning to gynecological disease screening. Among them, the “Digital Fertility Test Pen (2-in-1 HCG+LH)” integrates early pregnancy (HCG) and ovulation (LH) testing, providing results in 4 minutes with precise LED indicator prompts. Early pregnancy testing enables pregnancy detection 5 days in advance, featuring clear gestational week prompts and exceptional accuracy. The “Vaginitis 3-in-1 Test Card (3-in-1 CA+TV+GV)” adopts immunochromatographic technology, which can simultaneously detect three common pathogenic bacteria with one sample, and provide results in 10 minutes without complex instruments, suitable for medical institutions at all levels. The “Vaginal Microecology 7-in-1 Assessment Card” achieves full-dimensional assessment by testing 7 biochemical indicators, providing results in 20 minutes and supporting dual-mode interpretation of color comparison card and digitalization, providing a scientific basis for precise intervention of vaginal diseases.
Gastrointestinal Disease Screening Area: Non-Invasive Early Screening for Gastrointestinal Health
Addressing the pain points of “difficult early screening and cumbersome operation” of gastrointestinal diseases, the section launched two 3-in-1 test cards to achieve non-invasive, fast and accurate screening. The “Helicobacter Pylori + Fecal Occult Blood + Transferrin 3-in-1 Test Card (3-in-1 HP+FOB+TF)” only requires one fecal sample and can complete dual screening of Helicobacter pylori infection and gastrointestinal bleeding in 5 minutes, avoiding the inconvenience of traditional gastroscopy and breath test, and suitable for primary physical examination and family screening. The “Fecal Occult Blood + Transferrin + Calprotectin 3-in-1 Test Card (3-in-1 FOB+TF+CALP)” focuses on early screening of inflammatory bowel disease and gastrointestinal tumors. It also requires one-step operation and provides results in 5 minutes. With three advantages of good privacy, convenient operation and controllable cost, it provides an efficient solution for gastrointestinal health management.
Respiratory Pathogen Detection Area: Multi-Test in One Well, Fast, Accurate and Stable
Facing the clinical challenge of “diverse pathogens and difficult identification” in respiratory infections, Testsealabs brought the “one-well multi-test” detection product, achieving a technological breakthrough of “one sample, multiple pathogens detected simultaneously”. The product can be customized into multiple detection combinations such as 3-in-1 (influenza A + influenza B + COVID-19), 5-in-1 and 7-in-1 according to needs. Detection can be completed with only one sample well. Adopting immunochromatographic technology, it provides clear results in 10 minutes, helping clinicians quickly distinguish infection types, accurately formulate treatment plans, and greatly improve diagnosis and treatment efficiency.
Deepen Global Cooperation and Jointly Build a Healthy Future
A relevant person in charge of Testsealabs said: “As a weathervane of the global medical industry, MEDICA is an important window to showcase China’s IVD technological strength and a key platform to deepen global cooperation. By showcasing and communicating our core products at this exhibition, we hope to work with global medical colleagues to bring precise testing technology to more patients and jointly build a ‘smart interconnection · healthy future’.”
The exhibition will last until November 20th. Global industry partners are welcome to visit Booth 3H19 and explore the innovation path of in vitro diagnostics with Testsealabs.
About Testsealabs
Founded in November 2015, Hangzhou Testsea Biotechnology Co., Ltd. (Testsealabs for short) is a national high-tech enterprise focusing on the R&D, production and sales of in vitro diagnostic reagents. Headquartered in Yuhang District, Hangzhou, it is a key “large enterprise and group” cultivated by the district. It has also won qualification certifications such as Zhejiang “Specialized, Refined, Distinctive and Innovative” Enterprise and Zhejiang Provincial Enterprise Research Institute. It is moving forward steadily in the field of in vitro diagnostics with continuous technological innovation.






