Testsealabs FLU A/B + COVID-19/HMPV+RSV Antigen Combo Test Cassette (Nasal Swab)

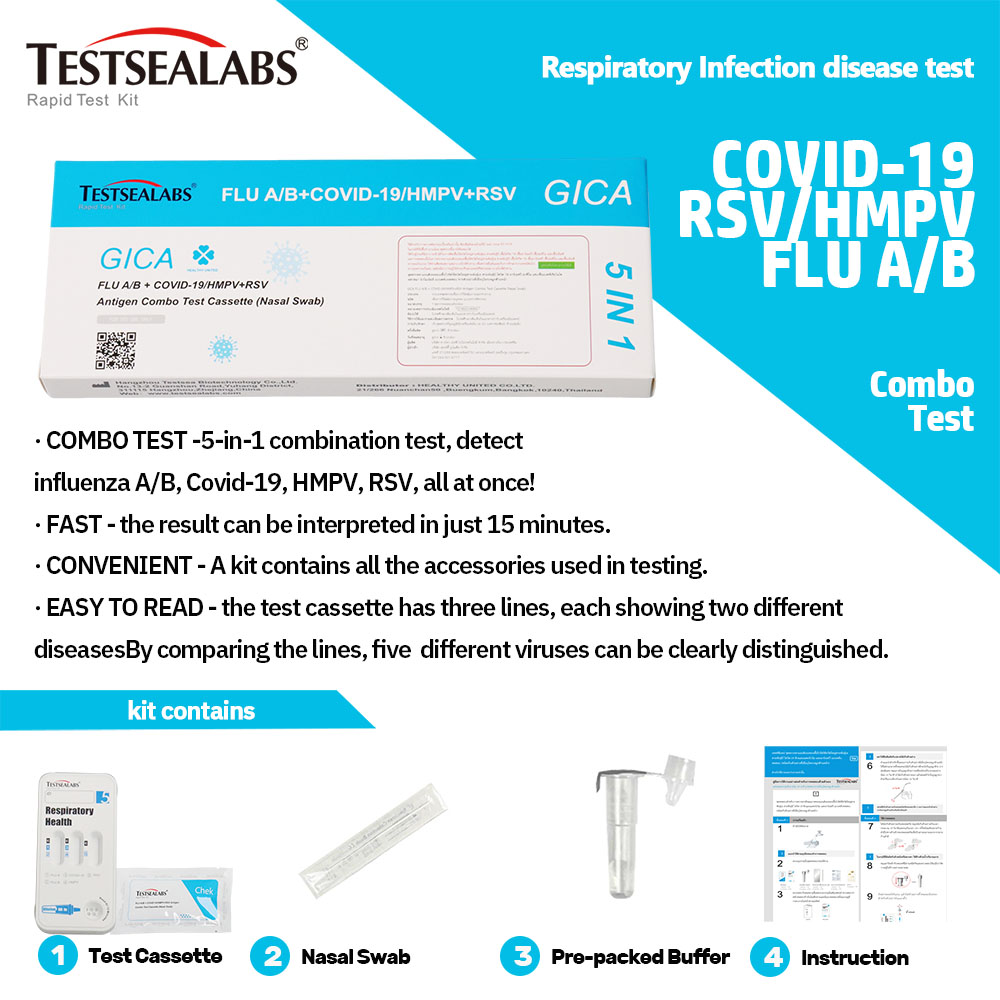
Product Name: FLU A/B + COVID-19/HMPV+RSV Antigen Combo Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of influenza A virus, influenza B virus, COVID-19, human metapneumovirus and respiratory syncytial virus antigen in nasal swab specimens.
FLU A/B + COVID-19/HMPV+RSV Antigen Combo Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of influenza A virus, influenza B virus, COVID-19, human metapneumovirus and respiratory syncytial virus antigen in nasal swab specimens.
Product usage scenarios
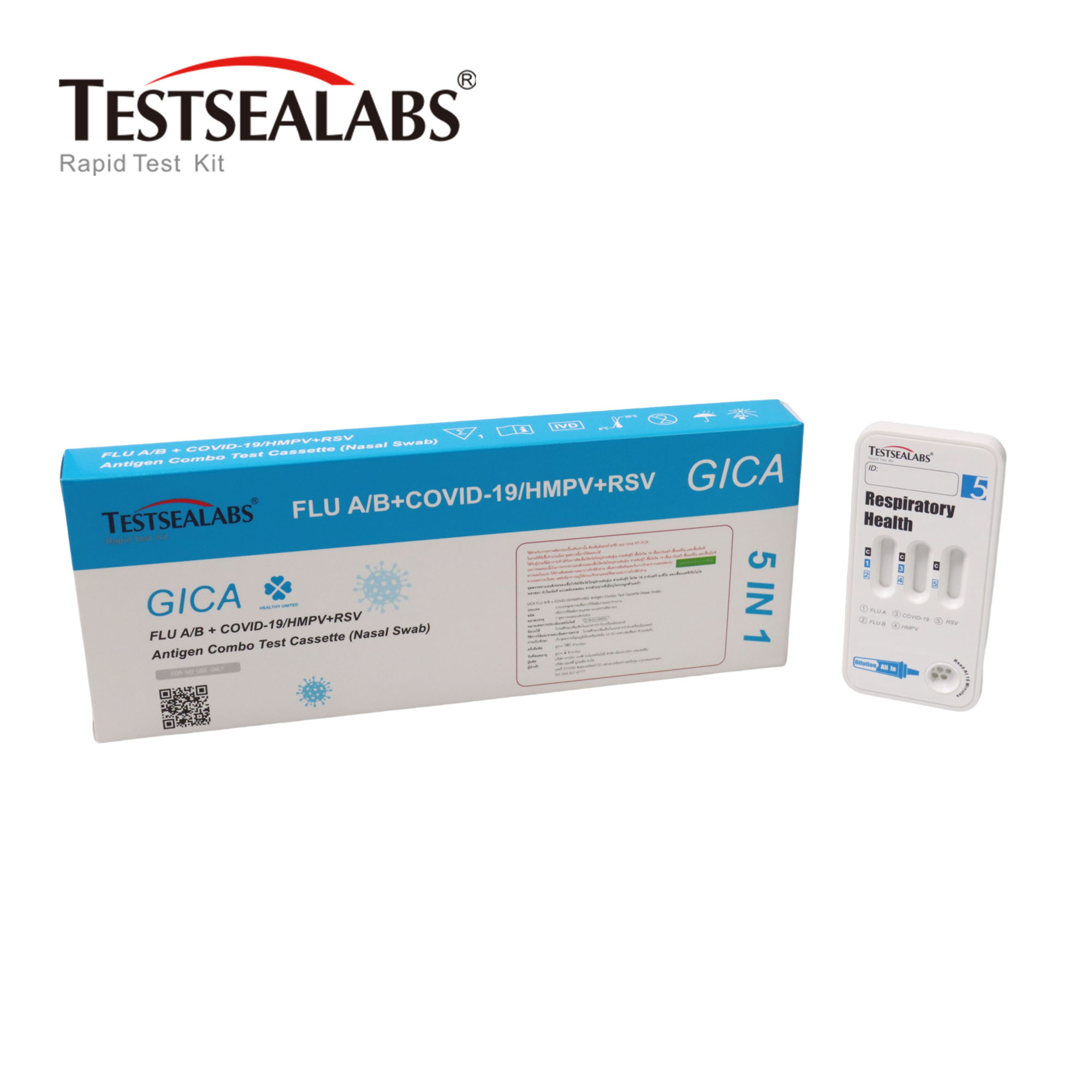
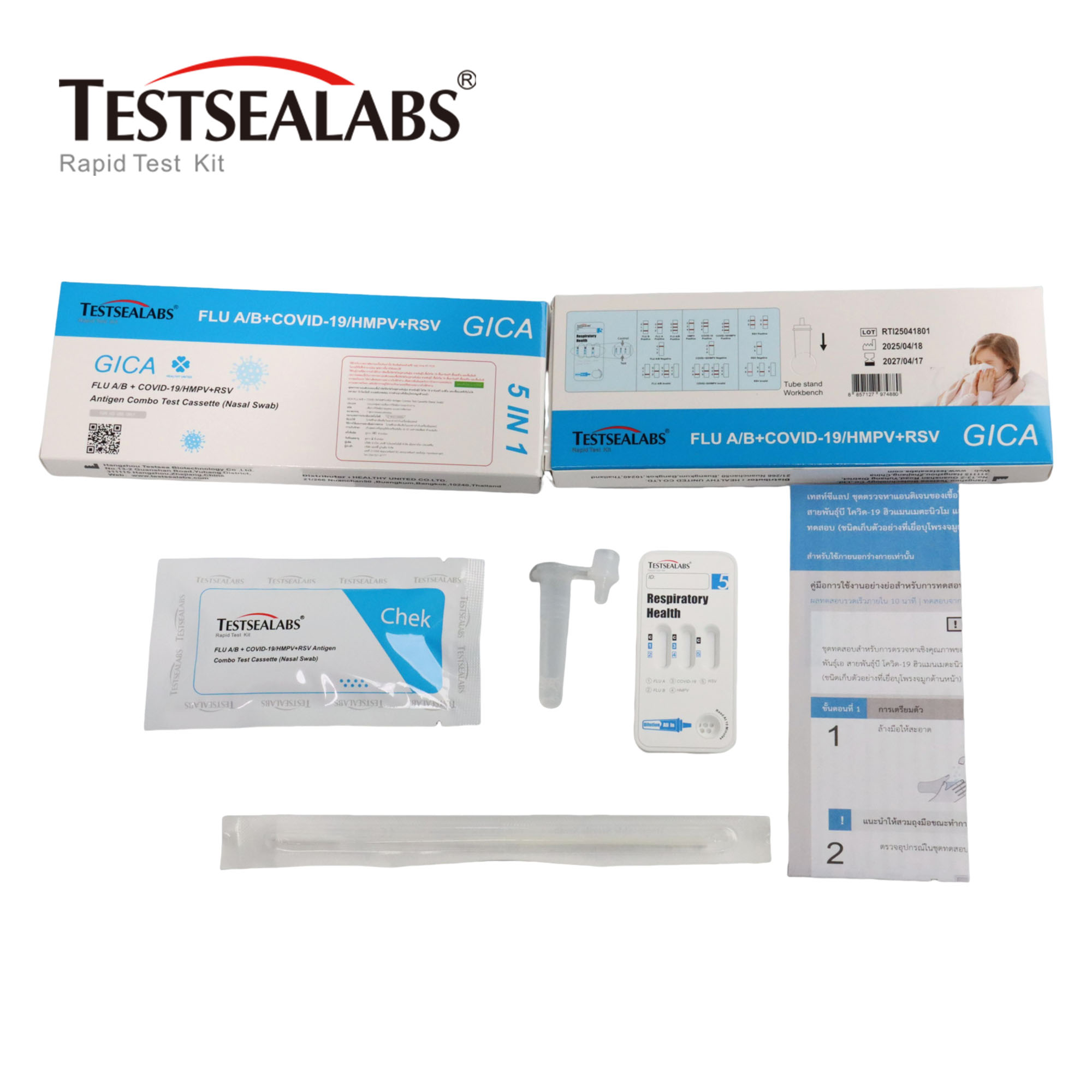


The FLU A/B + COVID-19/HMPV+RSV Antigen Combo Test Cassette is a qualitative membrane strip-based immunoassay for the detection of influenza A virus, influenza B virus, COVID-19 virus, human metapneumovirus and respiratory syncytial virus antigen in nasal swab specimens.
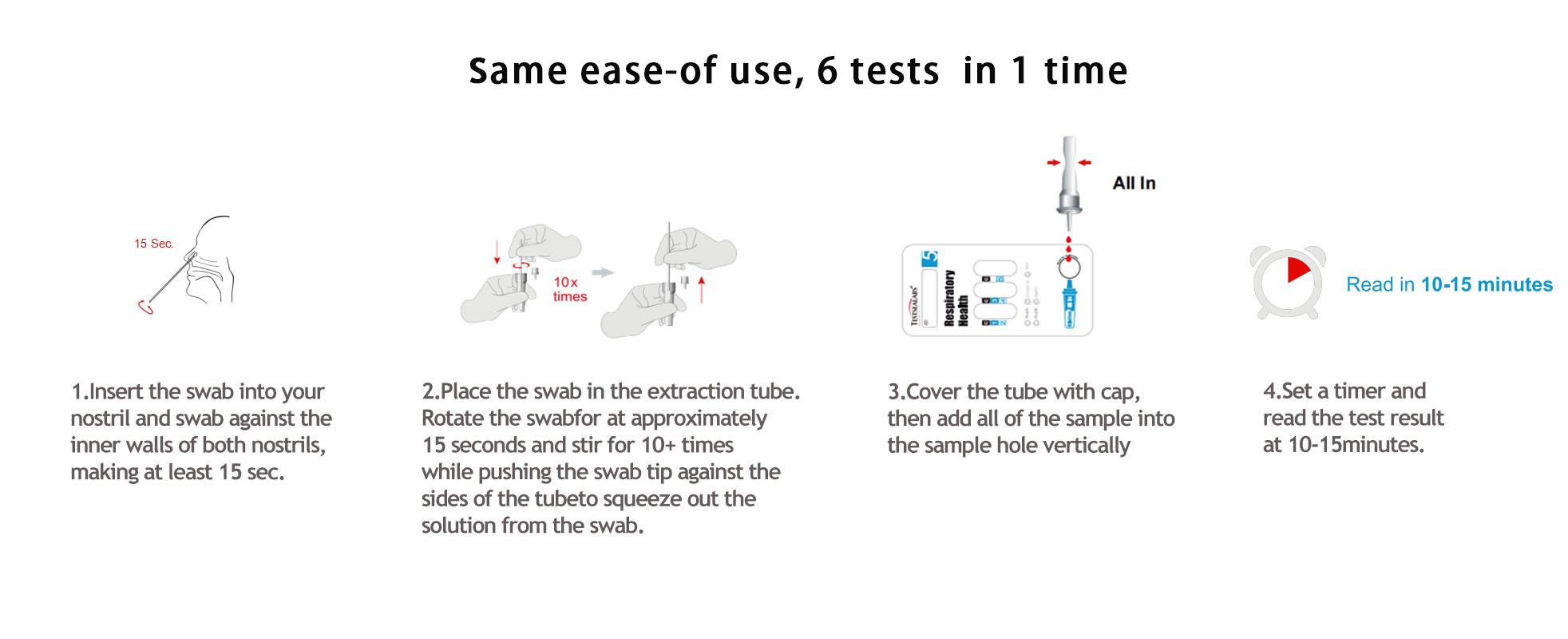
Interpretation of Results
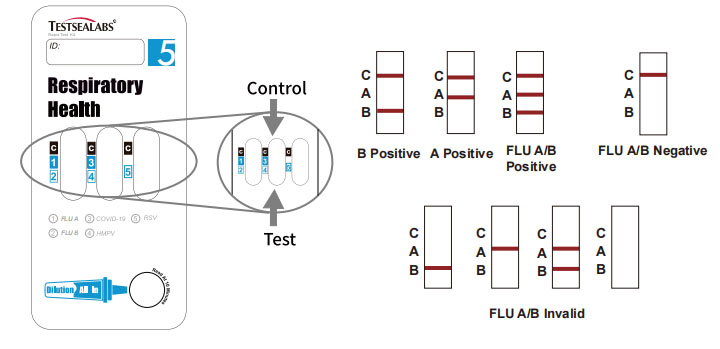
Positive: Control line and at least one test line appear on the membrane. The appearance of A test line indicates the presence of FLU A antigen. The appearance of B test line indicates the presence of FLU B antigen. And if both A and B line appear, it indicates that the presence of both FLU A and FLU B antigen. Lower the antigen concentration is, the weaker the result line is.
Negative: One colored line appears in the control region (C). No apparent colored line appears in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
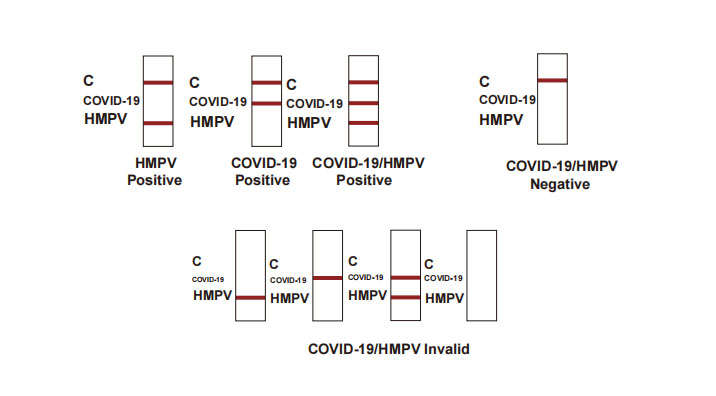
Positive: Control line and at least one test line appear on the membrane. The appearance of COVID-19 test line indicates the presence of COVID-19 antigen. The appearance of HMPV test line indicates the presence of HMPV antigen. And if both COVID-19 and HMPV line appear, it indicates that the presence of both COVID-19 and HMPV antigen. Lower the antigen concentration is, the weaker the result line is.
Negative: One colored line appears in the control region (C). No apparent colored line appears in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
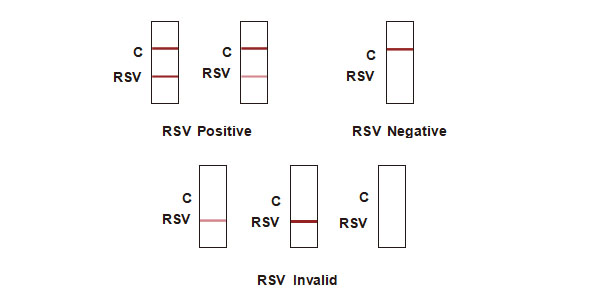
Positive: Control line and test line appear on the membrane.
Negative: One colored line appears in the control region (C). No apparent colored line appears in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
After-Sales Service Commitment
We provide comprehensive online technical consultations to address inquiries related to product usage, operational standards, and result interpretation. Additionally, customers may schedule on-site guidance from our engineers (subject to prior coordination and regional feasibility).
Our products are manufactured in strict compliance with the ISO 13485 quality management system, ensuring consistent batch stability and reliability.
After-sales concerns will be acknowledged within 24 hours of receipt, with corresponding solutions provided within 48 hours. A dedicated service file will be established for each customer, enabling regular follow-ups on usage feedback and continuous improvement.
We offer tailored service agreements for bulk purchasing clients, including but not limited to exclusive inventory management, periodic calibration reminders, and other personalized support options.
FAQ
yes, sure, we can provide the free samples.
Just feel free to contact us, send the quantity and products name to us, then we will give the quotation to you.
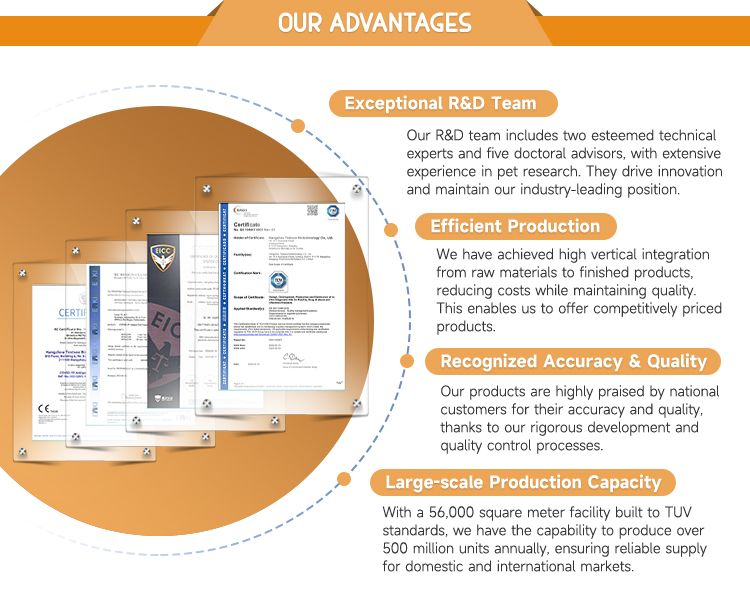
High-tech enterprise , specialized in the research, development productio and sales of raw materials, more than 56000 square
meters including 2000 square meters of GMP100 000-level purification workshop , follow with ISO management system.
Professional R & D team has more than 10 years experience.
With CE & ISO certificates.
Yes. We can accept OEM service. Meanwhile it's also welcome to choose our ODM products.
Company Profile