Testsealabs Disease Test Dengue IgG/IgM Rapid Test Kit
Product usage scenarios
The Dengue IgG/IgM Test is a rapid chromatographic test that detects antibodies (IgG and IgM) to the dengue virus in whole blood/serum/plasma. This test is useful aid in the diagnosis of dengue viral.
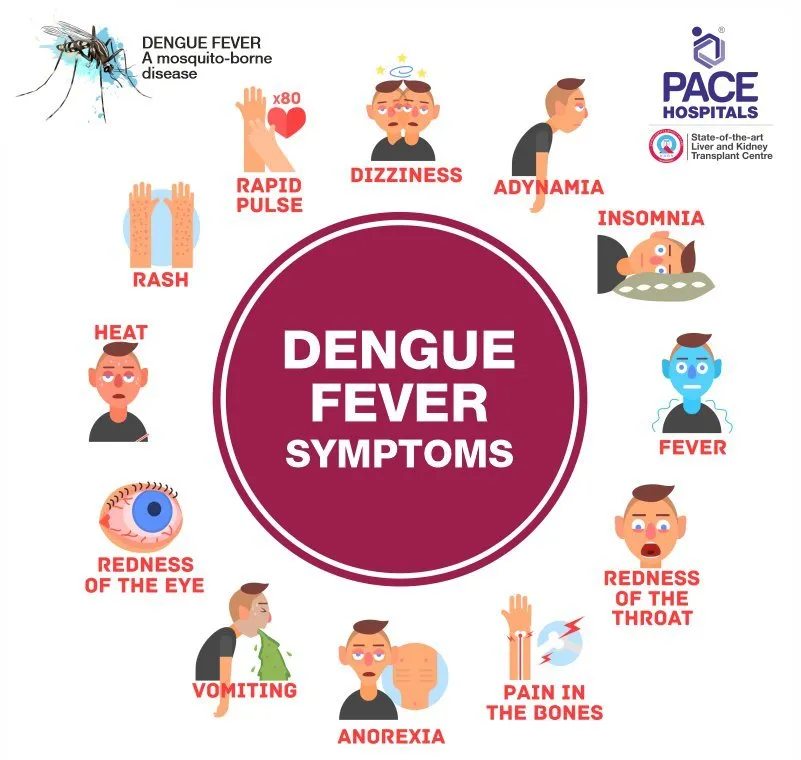
Dengue is transmitted by the bite of an Aedes mosquito infected with any one of the four dengue viruses. It occurs in tropical and subtropical regions of the world. Symptoms usually appear 3—14 days after the infective bite. Dengue fever is a febrile illness that can affect infants, young children, and adults. Dengue hemorrhagic fever, characterized by fever, abdominal pain, vomiting, and bleeding, is a potentially lethal complication that mainly affects children. Early clinical diagnosis and careful clinical management by experienced physicians and nurses can increase patients’ chances of survival.
The Dengue IgG/IgM Test is a simple and visual qualitative test that detects dengue virus antibody in human whole blood/serum/plasma.
The test is based on immunochromatography and can provide a result within 15 minutes.
Dengue fever continues to be a major global health concern, with over 1.4 million cases and 400 deaths reported in March 2025 alone. Early and accurate detection is essential in minimizing fatalities, particularly among older adults who are at greater risk of severe complications.
Real-life example: How early detection saved lives in dengue-prone regions
healthcare facilities in Southeast Asia implemented the Dengue IgM/IgG/NS1 Test to diagnose patients quickly during peak dengue seasons. This rapid diagnostic tool enabled medical teams to identify cases within 15 minutes, allowing for immediate treatment and reducing the burden on healthcare systems. Such initiatives have proven to be game-changers in regions where dengue fever is endemic.
Storage and Stability
Store the test in its sealed pouch at room temperature or refrigerated (4-30℃ or 40-86℉). The test device will remain stable until the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until it is used.
|
Materials |
|
|
Materials Provided |
|
| ●Test device | ●Buffer |
| ●Package insert | ●Disposable capillary |
|
Materials Required But Not Provided |
|
| ●Timer | ●Centrifuge Ÿ |
| ●Specimen collection container
|
|
Precautions
1. This product is intended for professional in vitro diagnostic use only. Do not use it after the expiration date.
2. Do not eat, drink, or smoke in the area where the specimens and kits are handled.
3. Handle all specimens as if they contain infectious agents.
4. Observe established precautions against microbiological hazards during all procedures and follow the standard procedures for proper disposal of specimens.
5. Wear protective clothing, such as laboratory coats, disposable gloves, and eye protection, when specimens are assayed.
6. Follow standard biosafety guidelines for handling and disposing of potentially infectious material.
7. Humidity and temperature can adversely affect results.
Specimens Collection and Preparation
1.The One Step Dengue Test can be performed used on Whole Blood /Serum / Plasma.
2.To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
3.Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens.
4.Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8 ℃ for up to 3 days. For long term storage, specimens should be kept below -20℃. Whole blood should be stored at 2-8 ℃ if the test is to be run within 2 days of collection. Do not freeze whole blood specimens.
5.Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
Interpretation of Results
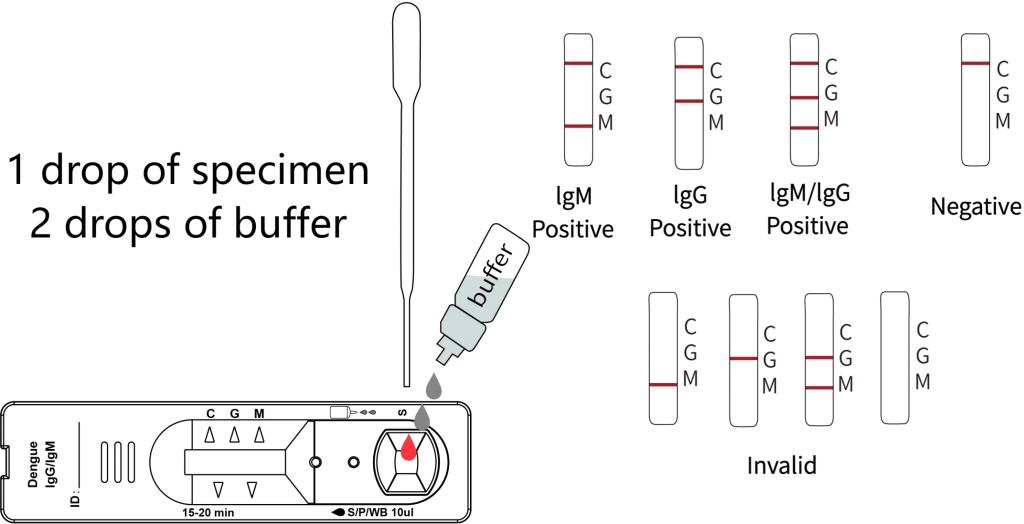
Positive: The control line and at least one test line appear on the membrane. The appearance of the G test line indicates the presence of dengue specific IgG antibody. The appearance of the M test line indicates the presence of dengue specific IgM antibody . If both G and M lines appear, it indicates that the presence of both dengue specific IgG and IgM antibody. The lower the antibody concentration, the weaker the result line.
Negative: One colored line appears in the control region(C). No colored line appears in the test line region.
Invalid: The control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.




