Testsealabs Disease Test Dengue IgG/IgM Rapid Test Kit
Precautions
1. This product is intended for professional in vitro diagnostic use only. Do not use it after the expiration date.
2. Do not eat, drink, or smoke in the area where the specimens and kits are handled.
3. Handle all specimens as if they contain infectious agents.
4. Observe established precautions against microbiological hazards during all procedures and follow the standard procedures for proper disposal of specimens.
5. Wear protective clothing, such as laboratory coats, disposable gloves, and eye protection, when specimens are assayed.
6. Follow standard biosafety guidelines for handling and disposing of potentially infectious material.
7. Humidity and temperature can adversely affect results.
Specimens Collection and Preparation
1.The One Step Dengue est can be performed used on Whole Blood /Serum / Plasma.
2.To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
3.Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens.
4.Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8 ℃ for up to 3 days. For long term storage, specimens should be kept below -20℃. Whole blood should be stored at 2-8 ℃ if the test is to be run within 2 days of collection. Do not freeze whole blood specimens.
5.Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
Interpretation of Results
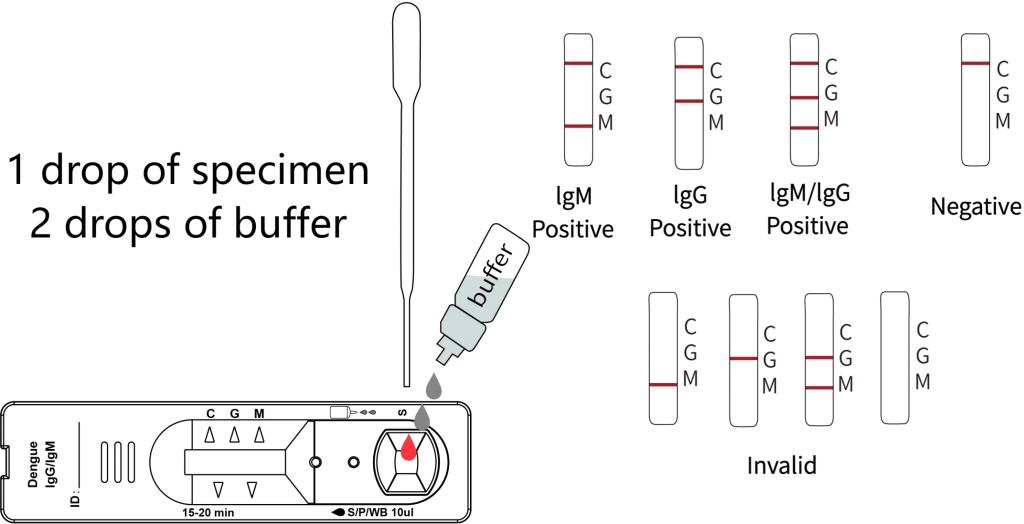
Positive: The control line and at least one test line appear on the membrane. The appearance of the G test line indicates the presence of dengue specific IgG antibody. The appearance of the M test line indicates the presence of dengue specific IgM antibody . If both G and M lines appear, it indicates that the presence of both dengue specific IgG and IgM antibody. The lower the antibody concentration, the weaker the result line.
Negative: One colored line appears in the control region(C). No colored line appears in the test line region.
Invalid: The control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
After-Sales Service Commitment
We provide comprehensive online technical consultations to address inquiries related to product usage, operational standards, and result interpretation. Additionally, customers may schedule on-site guidance from our engineers (subject to prior coordination and regional feasibility).
Our products are manufactured in strict compliance with the ISO 13485 quality management system, ensuring consistent batch stability and reliability.
After-sales concerns will be acknowledged within 24 hours of receipt, with corresponding solutions provided within 48 hours. A dedicated service file will be established for each customer, enabling regular follow-ups on usage feedback and continuous improvement.
We offer tailored service agreements for bulk purchasing clients, including but not limited to exclusive inventory management, periodic calibration reminders, and other personalized support options.




