Testsealabs Fecal Occult Blood+Transferrin+Calprotectin Antigen Combo Test
Fecal Occult Blood/Transferrin/Calprotectin Combo Test Cassette Instruction Manual
FOR PROFESSIONAL IN VITRO DIAGNOSTIC USE ONLY
Intended Use
Fecal Occult Blood/Transferrin/Calprotectin Combo Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of human hemoglobin, human transferrin and calprotectin in feces.
Summary
Many diseases can cause hidden blood in feces, known as fecal occult blood (FOB), human occult blood, or human hemoglobin. In the early stages, gastrointestinal disorders such as colon cancer, ulcers, polyps, colitis, diverticulitis, and fissures may have no obvious symptoms but only occult blood.
Transferrin (TF) is mainly present in plasma. It is almost undetectable in the feces of healthy people, but when gastrointestinal bleeding occurs, transferrin flows into the gastrointestinal tract and is excreted with feces, so it is abundant in the feces of patients with gastrointestinal bleeding.
Calprotectin is a calcium-zinc binding protein that acts as an acute inflammatory marker and is widely distributed in human cells, body fluids and tissues. Its functions include antibacterial activity, immune regulation and participation in the regulation of inflammatory response, and it has clinical application value in the diagnosis of infectious diseases, intestinal inflammation and cirrhosis complications.
Principle
This test is a qualitative membrane strip immunoassay for the detection of human hemoglobin, human transferrin and calprotectin in feces. During the test, antibodies against human hemoglobin, human transferrin and calprotectin are fixed in the test line area of the device.
After adding an appropriate amount of test sample to the sample hole, the sample reacts with the particles coated with antibodies against human hemoglobin, human transferrin and calprotectin on the sample pad. This mixture migrates along the test strip through chromatography and binds to the fixed antibodies against human hemoglobin, human transferrin and calprotectin.
If the sample contains human hemoglobin, human transferrin or calprotectin antigens, a colored line will appear in the test line area, indicating a positive result; if not, no colored line will appear in the test line area, indicating a negative result. As a procedural control, a colored line will always appear in the control line area, indicating that the sample volume is sufficient and the membrane has completed the wicking process.
Reagents
The test contains hemoglobin antibody, transferrin antibody and calprotectin antibody as capture reagents, and another batch of hemoglobin antibody, transferrin antibody and calprotectin antibody as detection reagents. Goat anti-mouse IgG antibody is used in the control line system.
Storage and Stability
- Store in the original sealed packaging at room temperature or refrigerated (4-30℃ or 40-86℉).
- The test device is stable until the expiration date printed on the sealed packaging.
- Do not open the sealed packaging until ready to use.
Materials
Materials Provided
- Test device
- Package insert
Materials Required But Not Provided
- Timer
- Specimen collection container
Precautions
- For professional in vitro diagnostic use only; do not use after expiration.
- Do not eat, drink or smoke in the area where samples and kits are handled.
- Treat all samples as if they contain infectious agents.
- Adhere to microbiological hazard prevention measures during all procedures and follow standard procedures for proper disposal of samples.
- Wear protective clothing (such as laboratory coats), disposable gloves and eye protection when testing samples.
- Follow standard biosafety guidelines for handling and disposing of potentially infectious materials.
- Humidity and temperature may affect test results.
Specimen Collection and Preparation
- This test can be performed on fecal samples.
- Remove the test device from the sealed packaging. Collect a random fecal sample in a clean, dry container. Open the extraction tube and use the sample collection applicator to randomly pierce the fecal sample at least five different positions. Do not scoop the fecal sample (this may lead to invalid test results), and avoid excessive fecal samples (this may also lead to invalid test results).
- If the sample is not tested within 6 hours, it can be stored at 2-8℃ for 3 days; for long-term storage, the sample should be stored below -20℃.
- Bring the sample to room temperature before testing. Frozen samples must be completely thawed and thoroughly mixed before testing; do not freeze and thaw samples repeatedly.
Test Procedure
Before testing, allow the test device, sample, buffer and/or controls to reach room temperature (15-30℃ or 59-86℉).
- Bring the packaging to room temperature before opening. Remove the test device from the sealed packaging and use it as soon as possible.
- Place the test device on a clean and level surface.
- Press and tighten the cap on the sample collection tube, then shake the sample collection tube vigorously to mix the sample and dilution buffer.
- Hold the sample collection tube upright, remove the limiter, and squeeze the extraction tube to allow the solution to flow out, then start timing.
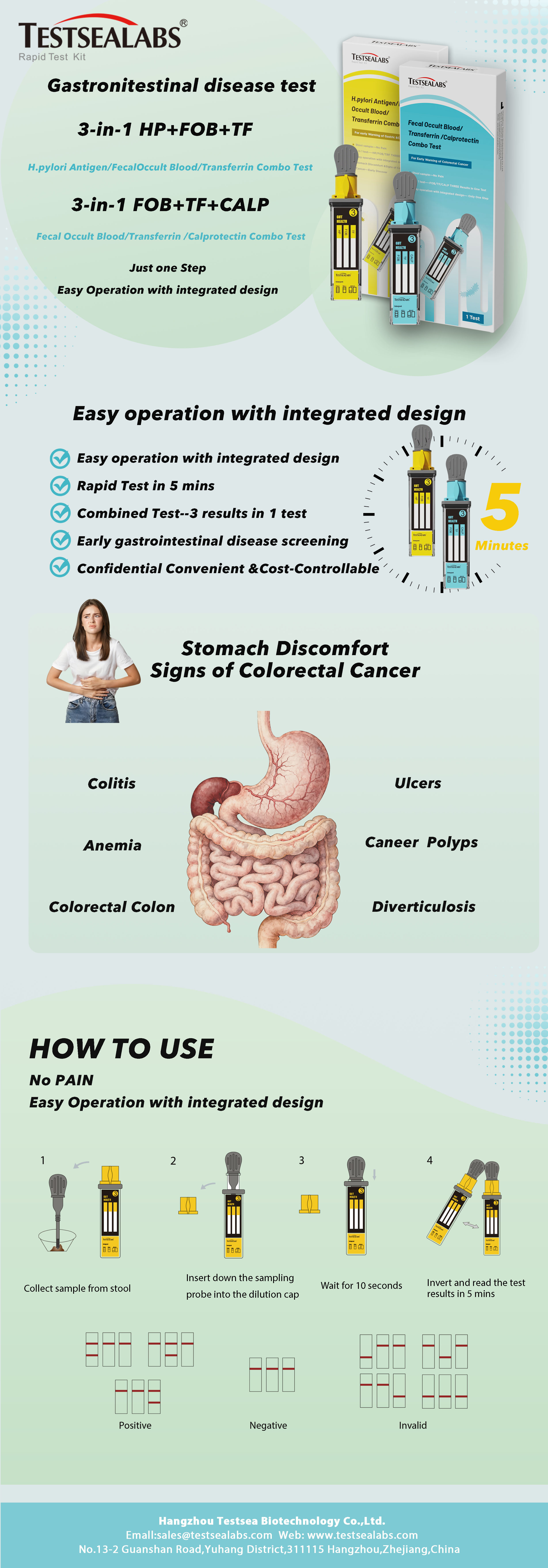
- Wait for the colored line(s) to appear. Read the result at 5 minutes; do not interpret the result after 15 minutes.
Interpretation of Results
- Positive: Two lines appear. One line always appears in the control line area (C), and another obvious colored line appears in the test line area.
- Negative: Only one colored line appears in the control line area (C); no obvious colored line appears in the test line area.
- Invalid: No line appears in the control line area. Insufficient sample volume or incorrect operation are the most likely causes of control line failure. Review the operation procedure and retest with a new test device. If the problem persists, stop using the test kit immediately and contact the local distributor.
Limitations
- This test is for in vitro diagnostic use only, and is only used for the detection of human hemoglobin, human transferrin and calprotectin in feces. This qualitative test cannot determine the quantitative value or increase rate of human hemoglobin, human transferrin and calprotectin.
- Like all diagnostic tests, all results must be interpreted in conjunction with other clinical information available to the physician.
- If the test result is negative but clinical symptoms persist, it is recommended to perform additional tests using other clinical methods.




