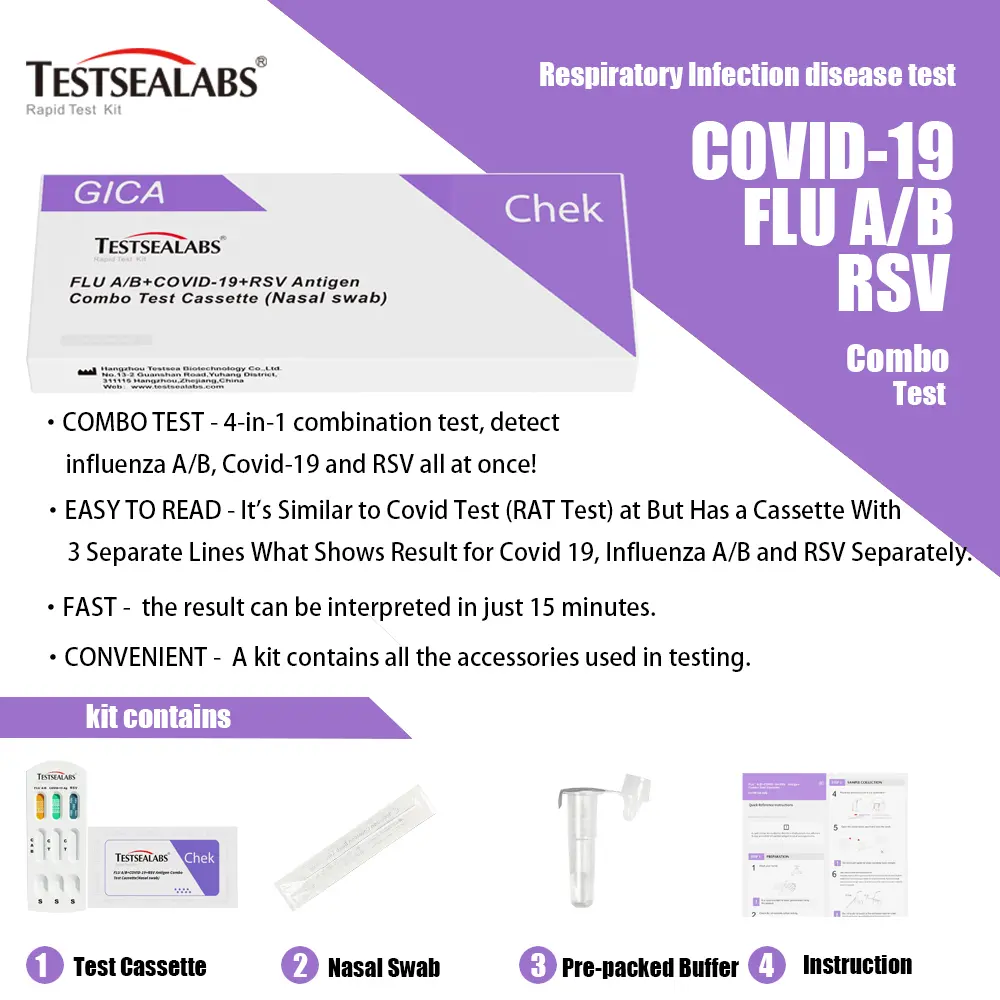
Testsealabs Influenza Ag B Test
Product Specification
| Principle | Chromatographic Immunoassay | Specimen | Nasopharyngeal Swab |
| Reading Time | 15 minutes | QMS Certification | ISO 13485 and MDSAP |
| Storage Temperature | 4-30°C | Shelf life | 2 years |
| Format | Cassette | Specification | 25T |
Intended Use
The Influenza Ag B Test is a rapid chromatographic immunoassay for the qualitative detection of influenza B virus antigen in nasopharyngeal swab specimens.
Summary
The Influenza Ag B Test is a rapid chromatographic immunoassay for the qualitative detection of influenza B virus antigen in nasopharyngeal swab specimens from individuals with suspected influenza B virus infection in conjunction with clinical presentation and the results of other laboratory tests. Results are for the detection of influenza B virus antigen. An antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Positive results indicate the presence of viral antigen, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out other bacterial/viral infection. The agent detected may not be the definite cause of disease. Negative results do not preclude influenza B infection and should not be used as the sole basis for treatment or patient management decisions. Negative results should be treated as presumptive and confirmed with a molecular assay, if necessary for patient management. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with influenza B virus. The Influenza Ag B Test is intended for use by trained clinical laboratory personnel.